Abstract
In this paper, an optimal semi-continuous process for vinegar production from edible alcohol through biotransformation by acetic acid bacteria (AAB) WUST-01 was developed. The optimized medium composition for the starting-up stage was glucose 5.1 g/L, yeast extract 26.2 g/L, and ethanol 11.9 mL/L, and the optimal ethanol for the following semi-continuous stage was 50 mL/L. In the semi-continuous biotransformation process, the optimal withdraw ratio was 50% of working volume with 12 h cycle time. With these conditions, the total acidity could reach to 77.3 g/L and the acidity productivity could reach to 3.0 g/(L h) in a 5 L reactor. Furthermore, it was investigated to strengthen vinegar synthesis through enhancing alcohol dehydrogenase and aldehyde dehydrogenase activity in AAB by ferrous ion and pueraria flower extract as the enzyme regulators. With these regulators, the vinegar synthesis efficiency can be improved 16.3 and 13.2% respectively.
Electronic supplementary material
The online version of this article (10.1007/s10068-017-0283-z) contains supplementary material, which is available to authorized users.
Keywords: Vinegar, Alcohol vinegar, Acetic acid bacteria, Vinegar synthesis regulatory
Introduction
Vinegar, that the main component is acetic acid, is a traditional acidic condiment which is originally used in cooking [1]. Nowadays, it has been widely applied in food industry, fish processing industry, home cleaning and air purification. The global demand for vinegar is significantly increasing in the last few years. In traditional, vinegar is generally produced by solid-fermentation using wheat, corn, soybeans and some other grain as raw material [2]. At present, directly transformation of edible alcohol to vinegar by acetic acid bacteria (AAB) provides an attractive alternative technology for vinegar production [3]. Comparing with the traditional production from grain, this technology possesses various advantages such as high efficiency, short producing cycle, almost the same rich flavor, high acidity, and low production cost.
Acetic acid bacteria (AAB) are gram-negative obligate aerobic bacteria, which can oxidize alcohols to the corresponding species of acids. AAB has a long history applied in fermentation industry [4]. In present, vinegar is mainly produced by semi-continuous biotransformation by AAB, i.e. a fraction of culture broth is withdrawn on a periodic basis and at the same time the same volume of fresh medium is supplemented into the reactor to keep constant working volume. In practice, there are two sequential stages for vinegar production. The first is starting-up stage which is mainly for AAB cell growth. The followed stage is semi-continuous biotransformation stage, which is the main vinegar production [3]. The vinegar production efficiency mainly depends on the process parameters of the two stages [5–11]. To find the optimal conditions for the two stages is significant to improve the vinegar production.
In vinegar production, the main biochemical process is biological oxidation, in which alcohol is partially oxidized to vinegar by AAB with two sequential enzymatic catalytic reactions, i.e. alcohol to acetaldehyde and acetaldehyde to acetic acid [3]. In this process, ethanol is oxidized to acetaldehyde by a pyrroloquinoline quinine-dependent alcohol dehydrogenase (ADH) and then acetaldehyde is further oxidized to acetic acid by an aldehyde dehydrogenase (ALDH) [12]. The enzymatic activity of ADH and ALDH directly affect the acetification reaction in AAB. So these enzymes are two ideal regulatory sites to enhance the vinegar productivity by AAB.
In this work, we optimized the medium formula for the start-up stage and semi-continuous biotransformation stage of vinegar production by AAB. Then a semi-continuous pilot process for vinegar production was simulated in a 5 L mechanical agitation tank fermenter. Furthermore, it was investigated to strengthen vinegar synthesis through enhancing ADH and ALDH activity in AAB.
Materials and methods
Microorganisms, Medium and Culture condition
The acetic acid bacteria, Acetobacter pasteurianus WUST-01, isolated from a brewing factory (Angel Yeast Co., Ltd) was applied in this work.
In this study, two kinds of media were used. The seeds medium YG1 (with composition of 10 g glucose, 10 g yeast extract, 0.5 g KH2PO4, 0.5 g MgSO4 and 0.5 g NaH2PO4, a certain amount edible alcohol (95% ethanol) and acetic acid in 1 L of distilled water) was used for seed culture. Fermentation medium YG2 (with composition of 5.1 g glucose, 26.2 g yeast extract, 0.5 g KH2PO4, 0.5 g MgSO4 and 0.5 g NaH2PO4, 50 g edible alcohol in 1 L of distilled water) was used for the semi-continuous biotransformation stage. Edible alcohol and acetic acid were added into medium before inoculating AAB. Yeast extract was purchased from Oxoid Ltd (Cheshire, England), and the other chemicals were purchased from Aladdin® reagent company (Shanghai, China).
A. pasteurianus WUST-01 was inoculated into Erlenmeyer flask (250 mL) with YG1 medium (50 mL) containing 10 g/L edible alcohol and 5 g/L acetic acid. It was cultured in a constant temperature shaker at 220 r/min and 30 °C for 48 h as the seeds culture.
Starting-up stage process
Both the starting-up stage process and semi-continuous biotransformation were carried out in a 5 L mechanical agitation tank fermenter whose maximum working volume was 4 L (BIOSTAT@ A plus, Sartorius, Germany). A starting-up process should be initiated before semi-continuous biotransformation. The goal of start-up stage is to achieve the sufficient large cell population for the subsequent acetification fermentation process in the possible shortest time. The fermenter was operated at 30 °C, 400 r/min stirring speed, 1 vvm aeration rate with 4 L of YG2 medium containing 20 g/L edible alcohol and 5 g/L acetic acid and 5% inoculation amount. To shorten the starting-up stage for improving the total process efficiency, the medium for the starting-up stage was optimized based on the YG2 medium composition through growth model optimization method with cell biomass (dry cell weight) as the optimization goal. The glucose concentration, yeast extract (as the nitrogen source) concentration and ethanol concentration in the YG2 medium were respectively optimized. The experimental conditions and method was given in the Supplementary material.
Semi-continuous biotransformation stage optimization
Semi-continuous biotransformation stage is the main stage for vinegar fermentation production, which was initiated from the end of starting-up stage. For the vinegar semi-continuous production, a fraction of culture broth is withdrawn on a periodic basis when alcohol was depleted to a certain concentration level, and the same volume of fresh medium was the supplemented into the fermentation system to start a new batch. The process was that after withdrawing a certain ratio of the total volume (such as 30%, v/v) a new batch cycle was carried out by adding the same volume of fresh YG2 medium with a high alcohol centration. Each repeated-batch was ended when the alcohol concentration was below 0.1 g/L. Each semi-continuous fermentation process was run five batch cycles. The fermenter was operated at 30 °C, 400 r/min stirring speed and with 2.0 vvm (air volume/culture volume min) aeration rate to provide sufficient oxygen for the acetification reaction. The alcohol centration in the supplementary medium and the withdrawal ratio are the key two factors to this semi-continuous biotransformation stage. To obtain a high productivity, the two factors were optimized. A series of withdrawal ratio, 30, 40, 50, and 60%, was investigated. And the range of alcohol centration was 30–70 g/L.
Strengthening vinegar production through regulating of ADH and ALDH in AAB
Alcohol dehydrogenase (ADH) and Aldehyde dehydrogenase (ALDH) are the two key enzymes for the acetification reactions of oxidizing alcohol to acetic acid [12]. If their activity could be improved, the acetic acid synthesis should be strengthened, and then the vinegar productivity could be further enhanced. The ferrous ion is the activator to ADH and ALDH [13]. Lu et al. [14] reported that pueraria flower extraction (PFE) is the main herbs of antialcoholic drug, which can regulate the activity of ADH and ALDH to improve alcohol catabolism in the human body. PFE maybe also could enhance the metabolism of ADH and ALDH in AAB. Therefore, we investigated the promote effect of Fe2+ (FeSO4·H2O) and PFE on the activity of ADH and ALDH in AAB. Also, the strengthening effect of vinegar production through enhancing the ADH and ALDH in AAB with Fe2+ and PFE was further investigated. The experiments were conducted in Erlenmeyer flask. The AAB seed broth was inoculated YG2 medium with 10% inoculation amount. The YG2 medium contain 20 g/L edible alcohol and a certain amount of FeSO4·H2O or PFE. To find the optimal concentration of the regulator, a series of FeSO4·H2O and PAE amount, 0.0, 10.0, 15.0, 20.0, 25.0, 30.0 mg/L, were investigated. It was cultured in a constant temperature shaker at 220 r/m and 30 °C for 48 h. Then ADH and ALDH in AAB were extracted and their enzymatic activities were assayed. The AAB biomass and acidity of the vinegar were also measured.
Analysis methods
The acidity of the culture broth was quantitatively determined by titration with 0.1 mol/L NaOH solution with phenolphthalein as pH indicator [8]. The alcohol content in culture broth was assayed by a biosensor analyzer (SBA 40E, Hayfield Ji’nan Science and Technology Development Co Ltd, Ji’nan China) with ethanol sensor. The total acid productivity was applied to indicate the rate of acid production, which was defined as the produced total acidity under unit time and unit volume. The yield means the conversion yield of alcohol to total acidity and is calculated as the following equation:
| 1 |
where CAcidity is the acidity in the culture broth (g/L), CAlcohol is the initial alcohol concentration in the medium (g/L), 1.304 is the molecular weight factor of acetic acid to ethanol.
The total biomass was determined by turbidity method based on the optical density measurements at 600 nm using a UV/visible spectrophotometer. The dry cell weight was calculated based on the relationship between the dry cell weight and OD600 nm.
The AAB cellular ADH and ALDH extraction and their activities assay were referred to the methods described by Adachi et al. [15, 16]. The AAB cell was collected from culture broth by centrifuge. Then the cell was lysed with sonication under ice bath. The supernatant was collected as the crude enzyme solution and the ADH and ALDH activity was assayed. Enzyme activity was assayed by the method of Adachi et al. [15, 16] that used ferricyanide as an electron acceptor. The rate of reduction of ferricyanide to ferrocyanide gave a quantitative amount of aldehyde oxidized. One unit of enzyme activity was defined as the amount of enzyme catalyzing the oxidation of 1 μmol of ethanol (or acetaldehyde) per min under these assay conditions and 4.0 absorbance unit equaled to 1 μmol of ethanol (or acetaldehyde) oxidized.
Results and discussion
Medium optimization for starting-up stage
The goal of start-up stage is to achieve sufficient large cell population for the subsequent acetification fermentation process within the possible shortest time. The medium composition is the key factor to the AAB growth rate. The content of the main medium component, i.e. glucose, yeast extract and alcohol, was optimized based on the growth curve with dynamic models methodology [17, 18]. In this methodology, growth curve was fitted with Logistic model to find the maximal biomass in theory (Xm) to the given concentration of this composition [18]. And a series of Xm to the corresponding concentration of this composition was further fitted with a peak function such as Gaussian function, Logistpk function, and Lorentz function to find the optimal value of this medium composition [17, 18]. At the optimal concatenation, the highest biomass can be obtained. The detail experimental data and the data processing were given in the Supplementary material.
The optimal concentration for glucose, yeast extract and alcohol were obtained. The experiment concentration and the optimal value of the corresponding composition were given in Table 1. The optimal composition (the YG2 main composition) was confirmed by measuring the AAB growth curve and it was also given in Supplementary material.
Table 1.
Experiment and the optimal concentration of the medium for start-up stage
| Medium component | Experiment concentration (g/L) | Optimal concentration (g/L) |
|---|---|---|
| Glucose | 2.0, 3.0, 4.0, 5.0, 6.0 | 5.1 |
| Yeast extract | 23.0, 24.0, 25.0, 26.0, 27.0 | 26.2 |
| Alcohol | 0.0, 10.0, 20.0, 30.0, 40.0 | 11.9 |
Optimization of the semi-continuous production process
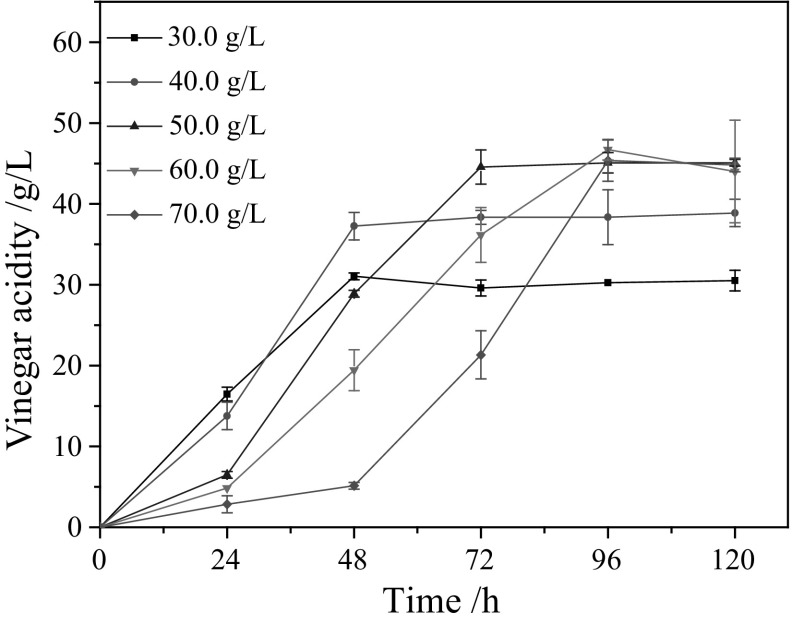
Semi-continuous production stage is the main stage for vinegar fermentation production. Edible alcohol is the main feedstock for the vinegar production. Its concentration is the key factor to control the product acidity and process efficiency. If the alcohol is insufficient, the process can’t reach a high productivity, and the vinegar acidity cannot meet the product standards. On the other hand, the excessively high alcohol concentration will inhibit the AAB activity and result in low productivity, even it causes the AAB death. So we optimized the alcohol concentration in the YG2 medium for the semi-continuous production process. Its concentration range from 30 to 70 g/L was investigated. The other medium composition in YG2 was not changed. The corresponding results were shown in Fig. 1.
Fig. 1.
Effect of alcohol concentrations on the vinegar acidity
As shown in Fig. 1, acidity increased along with the increase of alcohol concentration when the alcohol centration was lower than 50 g/L. At 50 g/L of alcohol, the acidity could reach to 45.0 g/L in 72 h and the yield was about 84.6%. Although the acidity could reach to 46.0 g/L with 60 g/L of alcohol centration, but it required longer time (i.e. 96 h) to convert all of the alcohol. And the yield is just only about 60%. It means a lower yield and productivity. Based on the productivity and yield, 50 g/L alcohol in YG2 is favorable to the practical production process.
In semi-continuous batch fermentation, the withdraw ratio is an important parameter which control the fermentation efficiency. It could change the microbial growth rate and vinegar productivity. We studied the semi-continuous batch fermentation with different withdraw ratios (30, 40, 50, and 60%) in 5 L pilot fermenter. Each batch was carried out five cycles.
The production curves for various withdraw ratios were given in Fig. 2. The results indicated that the total acidity all could reach to about 75.0 g/L with the four withdraw ratios. Even to the 40% of withdraw ratio, the total acidity could reach to 79.1 g/L. The corresponding yield were respectively 38.9, 60.3, 70.0, and 81.6% with 30, 40, 50, and 60% withdraw ratio. The required period length for each cycle to reach the stable acidity were respectively 11, 11, 12, and 16 h corresponding with 30, 40, 50, and 60% withdraw ratios. The highest acid productivity could be achieved with 50 withdraw ratio, which was about 3.0 g/(L h), and the total acidity could reached 77.3 g/L at the end of each cycle. This means that a higher productivity can be achieved compared the reported 34% withdraw ratio [9, 19].
Fig. 2.

Semi-continuous vinegar fermentation curve with various withdraw ratios. (A) 30%, (B) 40%, (C) 50%, (D) 60%
Enhancing ADH and ALDH activity to strengthen vinegar production
Alcohol dehydrogenase (ADH) and Aldehyde dehydrogenase (ALDH) are the two key enzymes for vinegar. To strengthen the vinegar productivity, it is an effective route through improving ADH and ALDH enzyme activity. Ferrous ion (FeSO4·7H2O) and PFE were reported that they can improve ADH and ALDH activity. They were respectively applied to regulate ADH and ALDH enzyme activity in AAB and further to strengthen vinegar production. The improving efficiency of ADH and ALDH enzyme activity and the acidity by adding ferrous ion (FeSO4·7H2O) and PFE was investigated. The experiments were conducted in triple parallel experiments. The error bar was included in the figures. The results of enzymatic activity and acidity promoted efficiency by FeSO4 and PFE were respectively given in Figs. 3 and 4. Figure 3(A) showed that the activity of ADH and ALDH increased with the increase of FeSO4·7H2O amount. When FeSO4·7H2O was 20.0 mg/L, the enzymatic activity reached the highest value and became stable. At this concentration, the activity of ADH increased from 0.513 to 0.877 U/mL, and the activity of ALDH increased from 0.635 to 0.987 U/mL. Corresponding, the acidity also increased from 45 g/L to a higher value of 52.8 g/L, which was improved 0.173 fold compared to the control.
Fig. 3.
Influence of FeSO4 on ADH and ALDH enzymatic activity and vinegar fermentation. (A) ADH and ALDH activity; (B) biomass and vinegar acidity
Fig. 4.
Influence of PFE on ADH and ALDH enzymatic activity and vinegar fermentation. (A) ADH and ALDH activity; (B) biomass and vinegar acidity
The results of PFE to the AAB vinegar production are shown in Fig. 4. The results showed that PFE could significantly improve ADH activity. But to ALDH, the improving efficiency was very small. The ADH activity increased from 0.723 to 0.989 U/mL. At this time, the acid production also reached a higher value, to 51.3 g/L acidity, which was 13.2% higher than the control. It shows that for AAB, PFE mainly acts on the metabolism of alcohol dehydrogenase.
In conclusion, the medium composition is important to the start-up stage of vinegar production by AAB. The optimal medium composition for starting-up stage was optimized as glucose 5.1 g/L, yeast extract 26.2 g/L, and alcohol 11.9 mL/L. The optimal composition of ethanol in semi-continuous fermentation stage is 50 mL/L. The withdraw ratio was 50% with 12 h cycle time in semi-continuous fermentation stage. With these conditions, the total acidity could reach to 77.3 g/L and the productivity could reach to 3.0 g/(L h). Also, ferrous ion and PFE are effective promoter to the strengthen vinegar synthesis through improving ADH or ALDH. With these activators, the vinegar synthesis efficiency can be improved 17.3 and 13.2% respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The present work was financed by the National Natural Science Foundation of China (Grant No. 21376184), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry), Foundation from Educational Commission of Hubei Province of China (Grant No. D20121108) and the Innovative Team of Bioaugmentation and Advanced Treatment on Metallurgical Industry Wastewater.
References
- 1.Ho CW, Lazim AM, Fazry S, Zaki UK, Lim SJ. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017;221:1621–1630. doi: 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- 2.Torija M, Mateo E, Vegas C. Effect of wood type and thickness on acetification kinetics in traditional vinegar production. Int. J. Wine Res. 2009;1:155–160. [Google Scholar]
- 3.Maria G, Elena V, Matteo C. Aerobic submerged fermentation by acetic acid bacteria for vinegar proguction: Process and biotechnological aspects. Process Biochem. 2014;49:1571–1579. doi: 10.1016/j.procbio.2014.07.003. [DOI] [Google Scholar]
- 4.Natsaran S, Kazunobu M, Osao A, Ivo F, Jitka F. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015;33:1260–1271. doi: 10.1016/j.biotechadv.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Tesfaye W, Morales ML, Garcia PM, Troncoso AM. Wine vinegar: Technology, authenticity and quality evaluation. Trends Food Sci. Technol. 2002;13:12–21. doi: 10.1016/S0924-2244(02)00023-7. [DOI] [Google Scholar]
- 6.Jo Y, Baek JY, Jeong IY, Jeong YJ, Yeo SH, Noh B, Kwon JH. Physicochemical properties and volatile components of wine vinegars with high acidity based on fermentation stage and initial alcohol concentration. Food Sci. Biotechnol. 2015;24:445–452. doi: 10.1007/s10068-015-0059-2. [DOI] [Google Scholar]
- 7.Schleputz T, Buchs J. Investigation of vinegar production using a novel shaken repeated batch culture system. Biotechnol. Prog. 2013;29:1158–1168. doi: 10.1002/btpr.1752. [DOI] [PubMed] [Google Scholar]
- 8.Qi Z, Yang H, Xia X, Quan W, Wang W, Yu X. Achieving high strength vinegar fermentation via regulating cellular growth status and aeration strategy. Process Biochem. 2014;49:1063–1070. doi: 10.1016/j.procbio.2014.03.018. [DOI] [Google Scholar]
- 9.Qi Z, Dong D, Yang H, Xia X. Improving fermented quality of cider vinegar via rational nutrient feeding strategy. Food Chem. 2017;224:312–319. doi: 10.1016/j.foodchem.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 10.Qi ZL, Yang HL, Xia XL, Wang W, Yu XB. High strength vinegar fermentation by Acetobacter pasteurianus via enhancing alcohol respiratory chain. Biotechnol. Bioprocess Eng. 2014;19:289–297. doi: 10.1007/s12257-013-0727-0. [DOI] [Google Scholar]
- 11.De OL, Romero LE, Cantero D. Optimum starting-up protocol of a pilot plant scale acetifier for vinegar production. Food Eng. 2002;52:31–37. doi: 10.1016/S0260-8774(01)00082-6. [DOI] [Google Scholar]
- 12.Yakushi T, Matsushita K. Alcohol dehydrogenase of acetic acid bacteria: Structure, mode of action, and applications in biotechnology. Appl. Microbiol. Biotechnol. 2010;86(5):1257–1265. doi: 10.1007/s00253-010-2529-z. [DOI] [PubMed] [Google Scholar]
- 13.Lechardeur D, Cesselin B, Fernandez A, Lamberet G, Garrigues C, Pedersen M, Gaudu P, Gruss A. Using heme as an energy boost for lactic acid bacteria. Curr. Opin. Biotechnol. 2011;22:143–149. doi: 10.1016/j.copbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Li W, Ni Y. Studies on the effect of water extracts of 8 different Chinese herbal medicines on alcohol dehydrogenase activity. J. Tongji Univ. Med. Sci. 2002;23(1):23–24, 30. [Google Scholar]
- 15.Adachi O, Tayama K, Shinagawa E, Matsushita K, Ameyama M. Purification and characterization of particulate alcohol dehydrogenase from Gluconobacter suboxydans. Agric. Biol. Chem. 1987;42:2045–2056. [Google Scholar]
- 16.Adachi O, Tayama K, Shinagawa E, Matsushita K, Ameyama M. Purification and characterization of membranebound aldehyde dehydrogenase from Gluconobacter suboxydans. Agric. Biol. Chem. 1980;44:503–515. [Google Scholar]
- 17.Dorini FA, Cecconello MS, Dorini LB. On the logistic equation subject to uncertainties in the environmental carrying capacity and initial population density. Commun. Nonlinear Sci. Numer. Simul. 2016;33:160–173. doi: 10.1016/j.cnsns.2015.09.009. [DOI] [Google Scholar]
- 18.Liu JZ, Weng LP, Zhang QL, Xu H, Ji LN. A mathematical model for gluconic acid fermentation by Aspergillus niger. Biochem. Eng. J. 2003;14:137–141. doi: 10.1016/S1369-703X(02)00169-9. [DOI] [Google Scholar]
- 19.Qi Z, Yang H, Zhang L, Leng Y, Quan W, Wang W. Study on the technology of high-acidity rice vinegar sumberged fermentation. J. Food Sci. Technol. 2010;29:911–915. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.