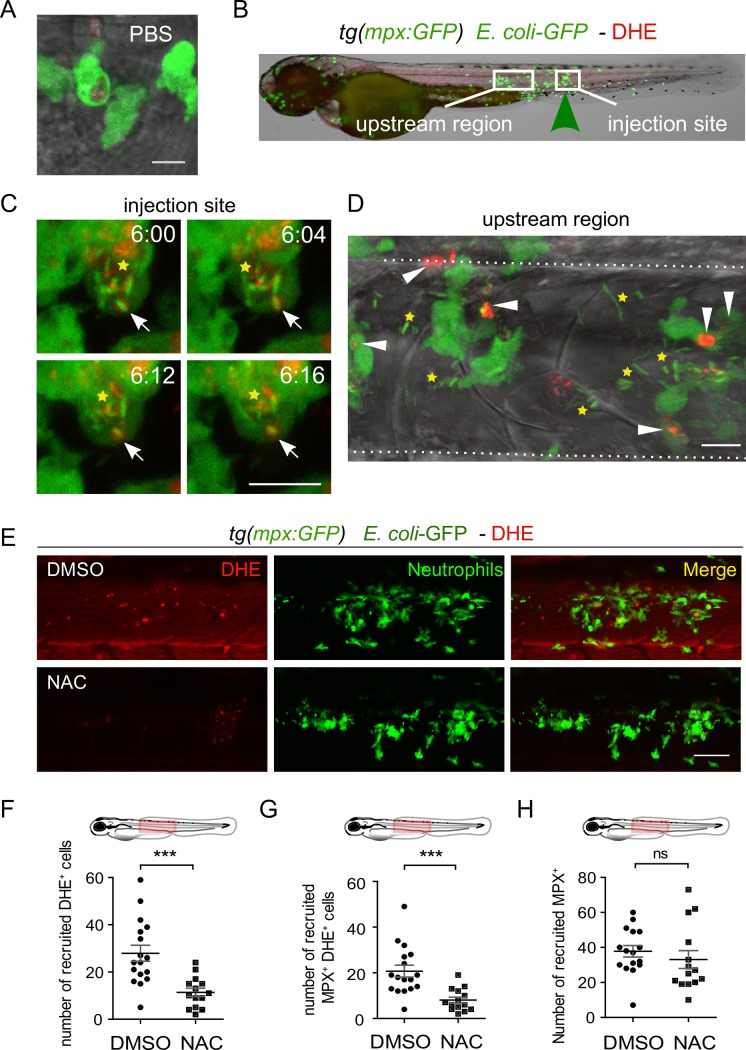
Fig 5. Superoxide is produced in neutrophils of infected larvae.
(A-D) Two dpf tg(mpx:GFP) embryos were either injected with PBS (A) or infected with E. coli-GFP in the notochord (B, C, D). At 6 hpi, superoxide was detected in living animals using Dihydroethidium (DHE, red) and neutrophils were visualized using GFP fluorescence (green). (A) Representative transmitted light images, overlaid with a maximal projection of confocal fluorescence images show that superoxide is lightly produced in the recruited neutrophil at the injection site. (B) White boxes in the larva image show the regions imaged by high resolution confocal microscopy and green arrowhead shows the injection site. (C) Representative time-lapse maximum projections starting 6 hpi during 16 min, show superoxide presence in phagosomes (white arrows) bearing bacteria (yellow stars: E. coli-GFP, Green) in recruited neutrophils at the injection site. Time is in minutes. (D) Representative transmitted light images, overlaid with a maximum projection of confocal fluorescence images show superoxide in neutrophils (white arrowheads) over the E. coli (yellow stars) infected notochord. Scale bars: 15 μm, dotted lines encase the notochord (NC). (E) Tg(mpx:GFP) larvae were infected with E. coli-GFP in the notochord and treated either with DMSO or NAC. Trunk images are representative maximum projections of single fluorescence (DHE and GFP) and merge channels using confocal microscopy. Scale bar = 50 μm. (F-H) Quantification of recruited DHE+ cells (F), recruited DHE+ MPX+ cells (G), and recruited neutrophils (H) in indicated conditions (mean number of cell/larva ± SEM, ***p<0.001, ns: non significant, NDMSO = 16–17 and NNAC = 13–14, from three independent experiments). The diagrams represent the regions selected for the counting.