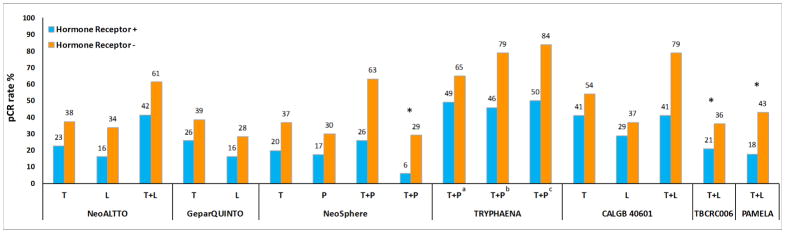
Figure 2. Rates of pathological complete response (pCR) by hormone receptor status of the primary tumor in published neoadjuvant trials of single or dual anti-HER2 therapy.
Abbreviations: pCR, pathological complete response; L, lapatinib; P, pertuzumab; T, trastuzumab. *, treatment arms without chemotherapy; a,b, treatment arms included 5-fluorouracil, epirubicin, cyclophosphamide, and docetaxel with different schedules; c, treatment arm included docetaxel and carboplatin. Numbers on top of the bars indicate pCR rate (%).