Figure 1. Molecular screens reveal regulators of antigen presentation as key factors in tumor sensitivity to immunotherapy.
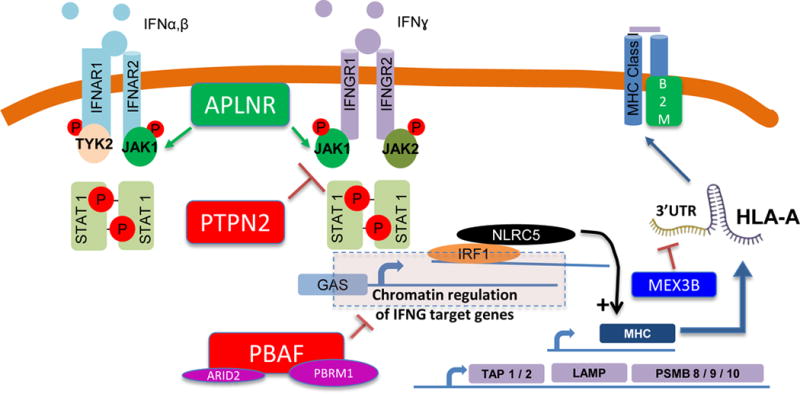
In the 1990s it was well documented that some patients who initially responded to cancer immunotherapies with interleukin-2 or tumor infiltrating lymphocyte adoptive cell transfer therapy may develop acquired resistance through loss of B2M, which leads to absence of surface expression of HLA class I. More recently, we identified defects in B2M from patients with acquired resistance to immune checkpoint blockade, along with defects in interferon signaling (JAK1, JAK2). Interferon signaling activates transcription of antigen processing machinery. Two in vitro and one in vivo CRISPR based screens have identified APLNR, PTPN2 and PBAF as immunotherapy targets, and each is a regulator of interferon signaling, and thereby each indirectly impacts downstream antigen presentation. The CRISPR screens also identified components of antigen processing machinery (e.g. TAP1, TAP2, and immunoproteasome subunits including PSMB9) as determinants of sensitivity to immunotherapy. NLRC5, a transcriptional regulator of MHC-I expression, has also been implicated in immunotherapy sensitivity in the CRISPR based screens. The work reported by Huang, et al. (1) in this issue of Clinical Cancer Research identifies MEX3B as a post-transcriptional regulator of HLA-A, via binding and disruption of the 3′UTR of the HLA-A mRNA.
