Figure 6.
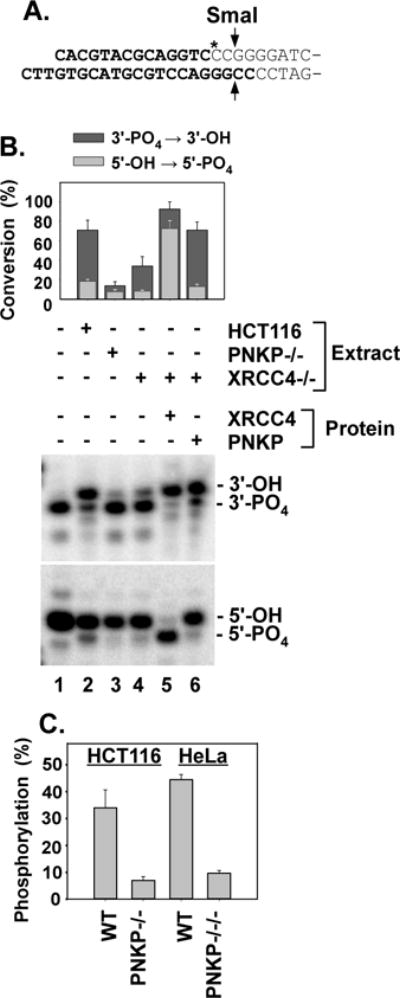
Dependence of 3′-phosphatase and 5′-kinase activities on XRCC4. A. Plasmid-length 5′-hydroxyl DSB substrate for 5′-phosphorylation. Bold lettering indicates oligomeric duplex that was ligated to pUC19. SmaI releases a labeled 16-base oligonucleotide. B. Upper gel: The 3′ overhang 3′-phosphate substrate (Fig. 4A) was incubated in HCT116 WT, XRCC4−/− or PNKP−/− extracts for 1 hr, cut with TaqαI and BstXI and 3′-dephosphorylation was assessed as in Fig. 3. Lower gel: The 5′-hydroxyl substrate shown in (A.) was incubated in the same extracts for 4 hr, cut with SmaI, and analyzed by gel electrophoresis to assess 5′-phosphorylation. C. The same substrate was incubated for 6 hr in whole-cell extracts of HCT116 and HeLa cells or their PNKP-deficient derivatives, and 5′-phosphorylation was similarly determined. Error bars show mean ± SEM for 3 experiments.
