Fig. 6.
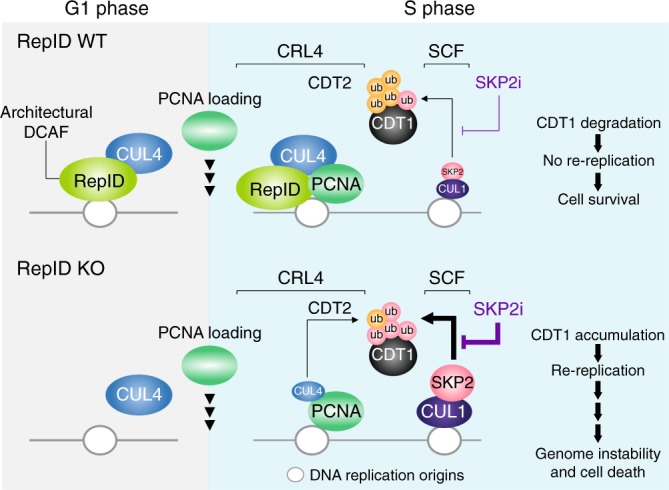
Model depicting RepID roles in regulating CRL4 activity and RepID-independent SCF functions during replication. In RepID wild-type (RepID WT) cells, CUL4 is recruited to chromatin by interacting with RepID (architectural DCAF) during G1 in the PCNA-free stage. During S-phase, PCNA is loaded and the CRL4-PCNA complex induces CDT1 ubiquitination using CDT2 (functional DCAF) by associating with early replication origins. Additional CUL4 can be recruited by PCNA during the G1/S transition and early S-phase. The CRL4-PCNA complex associates with CDT2 to degrade CDT1 later during S-phase. SCF is also recruited to prevent CDT1 accumulation at late replication origins. In cells expressing RepID, the SKP2 inhibitor has little effect because of the major role of CRL4 in degrading CDT1. In RepID depleted (RepID KO) cells, CUL4 loading to chromatin is compromised because RepID is not available to recruit it to chromatin during the G1 phase. During S-phase, some PCNA-dependent CUL4 is recruited, but CDT1 degradation is reduced because of low chromatin-bound CUL4 levels. Thus, in RepID KO cells, SCF recruitment to late replication origins compensates for the lack of CRL4 and have a greater role in targeting CDT1. Because SCF now acts as the major ubiquitin machinery for CDT1 degradation, the SKP2 inhibitor shows increased potency, and treatment leads to increased CDT1 levels and re-replication-mediated cancer cell death
