Introduction
Key Teaching Points.
-
•
Young patients with syncope, especially on exertion, should undergo provocative exercise testing.
-
•
Inflammatory cardiac disease might be the cause for transient complete heart block.
-
•
Cardiac magnetic resonance imaging is a suitable tool to monitor myocardial involvement on first presentation and during follow-up.
Cardiac sarcoidosis is a rare immunologic disease causing heart involvement in 5% of patients.1 Cardiac sarcoidosis may manifest clinically as a cardiomyopathy with impaired left ventricular (LV) function or as tachyarrhythmias or bradyarrhythmias.2 On autopsy, cardiac granulomas can be found in approximately 25% of patients. The most common location for granulomas and scars is the LV free wall, followed by the intraventricular septum, often with involvement of the conduction system.3, 4
Case report
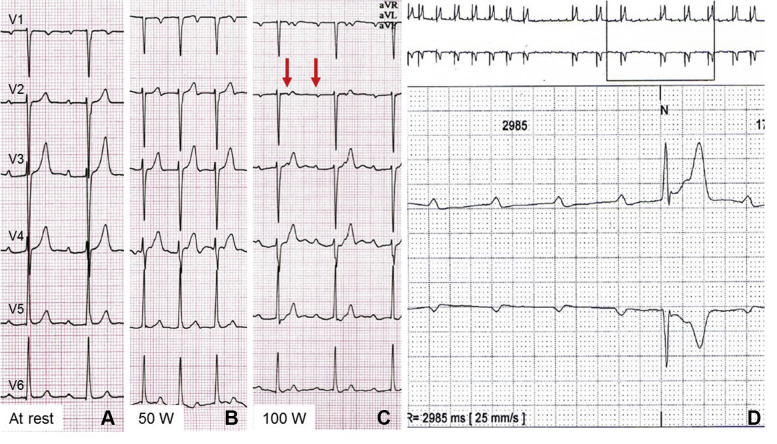
We report the case of a 34-year-old man presenting with a history of recurrent syncope on exertion. At rest, 12-lead electrocardiogram (ECG) showed first-degree atrioventricular (AV) block with undisturbed intraventricular conduction (Figure 1A). On exercise treadmill testing (Figure 1B), second-degree AV block with 2:1 AV conduction occurred with increasing workload at 100 W, suggesting infra-Hisian block (Figure 1C). Long-term ECG monitoring 1 day later demonstrated intermittent third-degree AV block (Figure 1D). Echocardiography showed both mildly impaired right ventricular (RV) and LV function (RV ejection fraction = 47%, LV ejection fraction [LVEF] = 54%) but normal diameters and mild tricuspid regurgitation. As the etiology of the underlying heart disease remained unclear, cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) was performed (Figure 2).
Figure 1.
Electrocardiogram at 25 mm/s paper speed at rest (A), at exercise 50 W (B), at exercise 100 W (C), and during Holter monitoring (D).
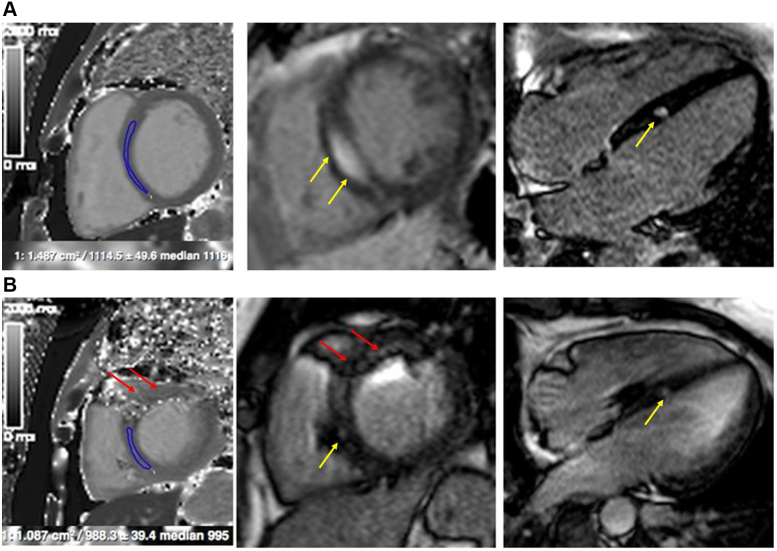
Figure 2.
Cardiac magnetic resonance on initial presentation (A) and during follow-up (B). Yellow arrows show late gadolinium enhancement, red arrows show pacemaker-induced artefacts.
CMR imaging revealed moderately reduced LV global systolic function (LVEF = 45%) with sharply demarcated intramyocardial enhancement within the mid-anterior septal segment, corresponding to the region of the bundle of His (Figure 2A). In addition, remote native T1 and T2 mapping values were elevated, consistent with the presence of myocardial inflammation. Together with bihilar lymphadenopathy on CMR, the findings were highly suggestive of cardiac involvement in a patient with systemic sarcoidosis. Subsequent bronchoalveolar lavage revealed an increased CD4/CD8 lymphocyte ratio, and lung and paratracheal lymph node biopsy provided the histologic proof of noncaseating granuloma. Subsequently, a 2-chamber pacemaker was implanted and the patient started on high-dose steroid therapy (1 mg per kg body weight per day).5 Two months after therapy initiation, pacemaker interrogation showed predominantly physiological AV conduction with only occasional need for ventricular pacing. On repeated CMR imaging, myocardial native T1 and T2 were almost normalized with less late gadolinium–enhanced myocardium (Figure 2B).
Discussion
Progressive AV-nodal conduction disturbances are a hallmark of cardiac sarcoidosis.1, 2 The present case illustrates the importance of a multidisciplinary approach to establishing the diagnosis of this disease entity. Collaboration between electrophysiologists, magnetic resonance imaging specialists, and pulmonologists enables a timely diagnosis and appropriate therapy.
Diagnosis
Cardiac sarcoidosis should be suspected in young and middle-aged patients (<50 years) presenting with unexplained syncope in the setting of AV conduction abnormalities and/or ventricular arrhythmias with or without heart failure.1, 2, 5 All patients with extracardiac sarcoidosis are recommended to be actively screened for cardiac involvement by electrocardiography and echocardiography.1, 5 Although there are no pathognomonic diagnostic criteria to date, abnormal thinning or thickening of the septal wall, wall motion abnormalities not corresponding to a coronary region, and systolic or diastolic dysfunction, as well as pericardial effusion, might be present in cardiac sarcoidosis.1, 2 If either a patient’s history or electrocardiography or echocardiography is positive for cardiac sarcoidosis, further diagnostic testing with CMR imaging is recommended owing to a Heart Rhythm Society consensus statement of 2014.5 Apart from myocardial thickening and/or wall motion abnormalities, enhanced T1 and T2 signal as markers for inflammation and edema4 can be found in the setting of sarcoidosis, especially in an early, clinically silent stage. Furthermore, LGE might visualize granuloma or scarring, often found in a patchy distribution.4 Compared to fluorodeoxyglucose positron emission tomography, CMR imaging was considered to be of better specificity but worse sensitivity. Newer observational studies found that an optimal magnetic resonance imaging technique adjudged by an expert cardiologist and radiologist can yield a sensitivity of 100% to diagnose cardiac sarcoidosis.1 Endomyocardial biopsy can confirm cardiac involvement in systemic sarcoidosis, but owing to the disseminated granulomatous infiltration, only 25% of cardiac biopsies in patients with cardiac sarcoidosis are positive.3 That is why extracardiac biopsies (eg, from pulmonary lymph nodes or lung tissue) are preferred in patients with systemic sarcoidosis. If only a cardiac manifestation is suspected, an image-guided endomyocardial biopsy (eg, with CMR or 3-dimensional voltage map) is recommended by current consensus statements.5 To establish the risk of sudden cardiac death, an electrophysiological study can be performed as a class IIb recommendation in patients with LVEF > 35%, despite optimal medical therapy and a period of immunosuppression, if there is still evidence of active inflammation.5 A small prospective study of 75 patients with cardiac sarcoidosis undergoing extensive programmed electrical stimulation demonstrated that event-free survival in patients with inducible ventricular arrhythmia on programmed electrical stimulation is significantly lower.
Treatment
Owing to the scarcity of data, it is still unknown whether only patients with myocardial inflammation and signs of active sarcoidosis or all patients with sarcoidosis, even in clinically silent stages, should be treated. There are no randomized, prospective data on treatment with corticosteroids. A recently initiated randomized controlled trial, the CASTOR study, had to be closed owing to a lack of funding. Small cohort studies report improvement in AV conduction as well as in LV function in patients with initially severely depressed LVEF (<35%), but no regeneration in patients with moderately impaired LVEF under immunosuppressive therapy with corticosteroids.1, 2 The current expert consensus paper recommends immunosuppression as a class IIa recommendation in patients with AV block type II (Mobitz) or higher as well as ventricular ectopy or (non-) sustained ventricular arrhythmia and/or evidence of active myocardial inflammation.5 Initially, a daily dose of 30–40 mg is recommended, requiring close monitoring. Treatment response should be evaluated after 1–3 months and steroid dose be tapered to 5–15 mg daily for 9–12 months.1 In cases of relapses, methotrexate has been successfully used to skimp steroid doses.1 In patients with depressed LV function, initiation of heart failure therapy including angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, or aldosterone antagonists might be useful. An implantable cardioverter-defibrillator (ICD) implantation is recommended as a class I indication for patients with cardiac sarcoidosis and documented either sustained ventricular arrhythmias, a history of survived cardiac arrest, or LVEF < 35%.5 An ICD can be useful (class IIa recommendation) in patients with unexplained syncope, indication for permanent pacing, or positive electrophysiological study.5 In patients with moderately impaired LV function (36%–49%) or impaired RV function (<40%), an ICD may be considered (class IIb recommendation) if signs of active myocardial inflammation persist despite immunosuppression and optimal heart failure therapy.5 In the latter group, a temporary protection with the wearable cardioverter-defibrillator might be indicated during risk stratification.
Prognosis
Cardiac sarcoidosis is generally associated with a less favorable outcome in patients with systemic sarcoidosis.1, 2, 3, 4, 5 Nonetheless, a recent Finnish study demonstrated a 10-year survival of 92.5% in patients with cardiac sarcoidosis owing to modern heart failure therapy including active transplant surgery.1 The magnitude of LV dysfunction is considered to be a strong prognostic marker.1, 2, 4 With rising CMR technology and research, the extent of myocardial LGE might be a more important prognostic factor compared to LV function.1 Native T1 and T2 values proved to be excellent screening markers in patients with systemic sarcoidosis and silent cardiac involvement.4
Conclusion
Although cardiac sarcoidosis is a rare disease, young people with a history of syncope and complete heart block should be actively screened to initiate early treatment. CMR imaging seems to be a suitable, noninvasive diagnostic tool to monitor myocardial involvement.
References
- 1.Birnie D.H., Kandolin R., Nery P.B., Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38:2663–2670. doi: 10.1093/eurheartj/ehw328. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 3.Uemura A., Morimoto S., Hiramitsu S., Kato Y., Ito T., Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–330. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 4.Puntmann V.O., Isted A., Hinojar R., Foote L., Carr-White G., Nagel E. T1 and T2 mapping in recognition of early cardiac involvement in systemic sarcoidosis. Radiology. 2017;285:63–72. doi: 10.1148/radiol.2017162732. [DOI] [PubMed] [Google Scholar]
- 5.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]