Figure 1.
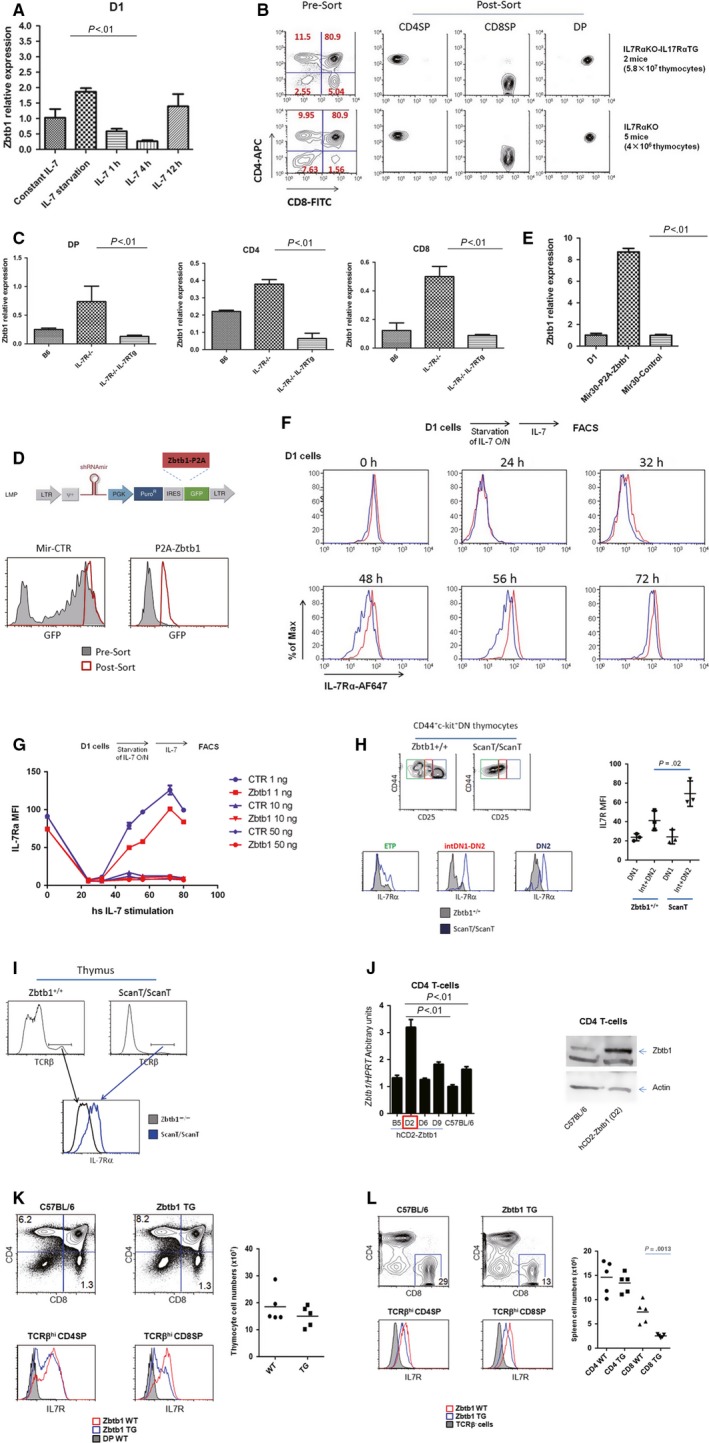
Zbtb1 and IL‐7Rα signalling mutually regulate each other both in vitro and in vivo. A, D1 cells were maintained in complete medium with 10 ng/mL IL‐7 or deprived of IL‐7 overnight and re‐stimulated with IL‐7 for 1 h, 4 h and 12 h. Zbtb1 transcripts were analysed by RT‐qPCR in various conditions. HPRT were served as internal control. B, DP, CD4SP and CD8SP thymocytes were sorted from mice with different genotypes as indicated. C, Zbtb1 transcripts from thymocytes mentioned in (B) were detected by RT‐qPCR. D, D1 cells were transduced by retroviral vector Mir‐CTR or Zbtb1 overexpressing retrovirus Mir‐P2A‐Zbtb1. Stable cell lines were generated by sorting GFP + cells. E, Zbtb1 overexpression in D1 stable cell line was confirmed by RT‐qPCR. F, G, Two stable D1 cell lines with or without Zbtb1 overexpression were deprived of IL‐7 overnight and re‐stimulated with various concentration of IL‐7 for different period of time. Surface IL‐7Rα expression in each condition was detected by flow cytometry. The representative data of D1 cells re‐stimulated with 1 ng/mL IL‐7 after starvation were shown in (F). H, I, The surface IL‐7Rα expression of different subpopulation of thymocytes in wild‐type and ScanT mice were analysed by flow cytometry. J, The overexpression of Zbtb1 in the D2 line of hCD2‐Zbtb1 transgenic mice was confirmed by RT‐qPCR and Western blot. K, The total numbers of thymocytes between wild‐type and Zbtb1 transgenic mice were comparable. The surface IL‐7Rα expression in CD4SP and CD8SP thymocytes of wild‐type and Zbtb1 transgenic mice were evaluated by flow cytometry. L, The total numbers of CD4 and CD8 T cells in spleen were compared between wild‐type and Zbtb1 transgenic mice. The surface IL‐7Rα expression in CD4SP and CD8SP TCRβhi spleenocytes of wild‐type and Zbtb1 transgenic mice were evaluated by flow cytometry
