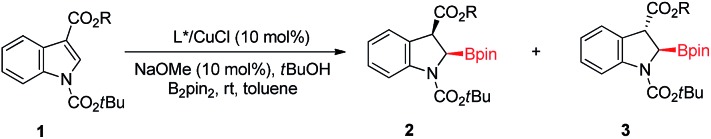
Table 1. Optimization of the reaction conditions for the asymmetric dearomative borylation a .
| |||||
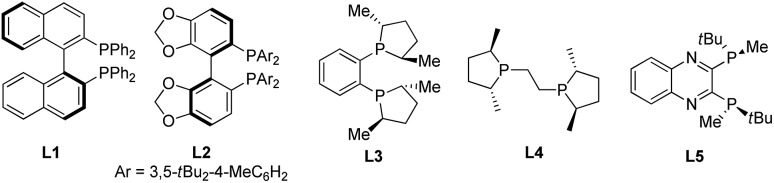
| Entry | Ligand | 1: R | Yield b (%) | d.r. c | ee d (%) |
| 1 | L1 | 1a: Me | Trace | n.d. | n.d. |
| 2 | L2 | 1a: Me | Trace | n.d. | n.d. |
| 3 | L3 | 1a: Me | 44 | 45 : 55 | 92 |
| 4 | L4 | 1a: Me | 60 | 80 : 20 | 80 |
| 5 | L5 | 1a: Me | 90 | 92 : 8 | 86 |
| 6 | L5 | 1b: Et | 86 | >98 : 2 | 91 |
| 7 | L5 | 1c: iPr | 93 | 94 : 6 | 94 |
| 8 | L5 | 1d: tBu | 72 | 97 : 3 | 81 |
| 9 e | L5 | 1c: iPr | 46 | 50 : 50 | 96 |
| 10 f | L5 | 1c: iPr | 55 | 63 : 37 | 97 |
| 11 g | L5 | 1c: iPr | 56 | 70 : 30 | 97 |
| 12 h | L5 | 1c: iPr | 85 | 95 : 5 | 95 |
| |||||
aUnless otherwise noted, all the reactions were carried out with 1 (0.2 mmol), L (0.02 mol), CuCl (0.02 mmol), NaOMe (0.02 mmol), alcohol (0.4 mmol), and B2pin2 (0.3 mmol) in toluene (1 mL) at 25 °C for 16 h.
bThe yield of isolated cis-product 2.
cThe diastereoselective ratio (cis/trans) was determined by 1H NMR of crude reaction mixtures.
dThe enantiomeric excess was determined by HPLC on a chiral IE column.
eMeOH was used instead of tBuOH.
fEtOH was used instead of tBuOH.
g iPrOH was used instead of tBuOH.
hThe reaction was carried out at 0 °C for 18 h.
