Table 2. Scope of the enantioselective allylic alkylation with regard to allylic carbonates a .
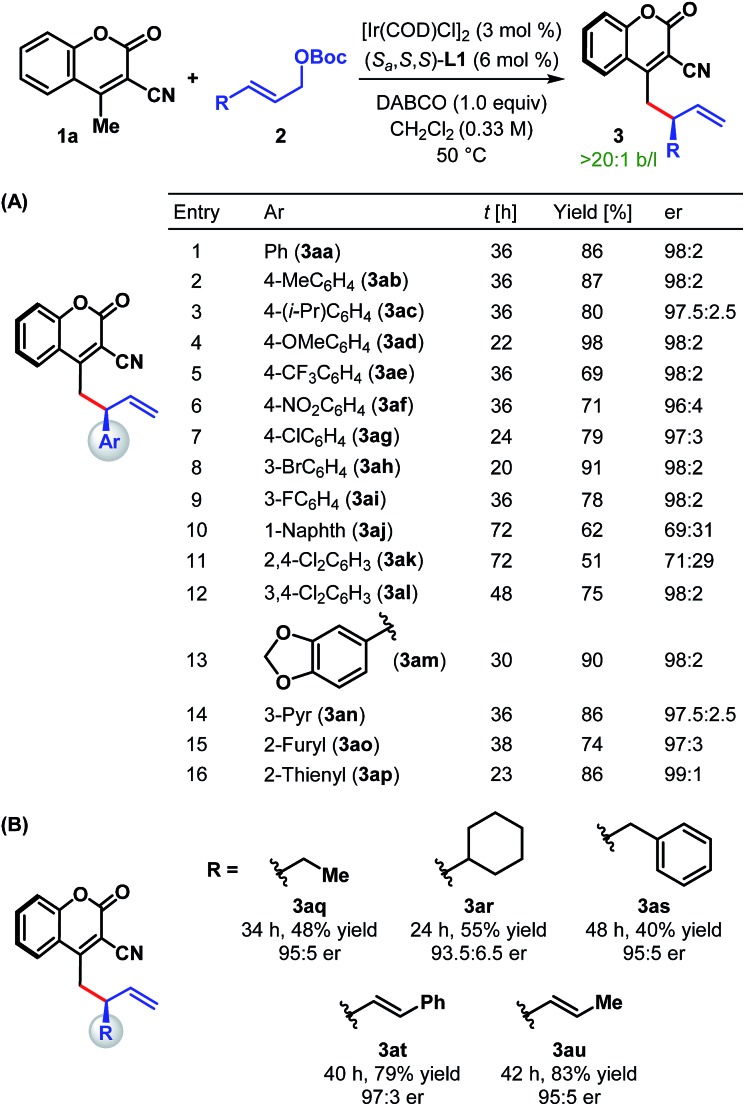
|
aReaction conditions: 3 mol% [Ir(COD)Cl]2, 6 mol% L1, 0.24 mmol of 1a, 0.2 mmol of 2 and 0.2 mmol of DABCO in 0.6 mL CH2Cl2. The catalyst was prepared via n-PrNH2 activation. Yields correspond to the isolated product after chromatographic purification. er was determined by HPLC analysis on a chiral stationary phase.
