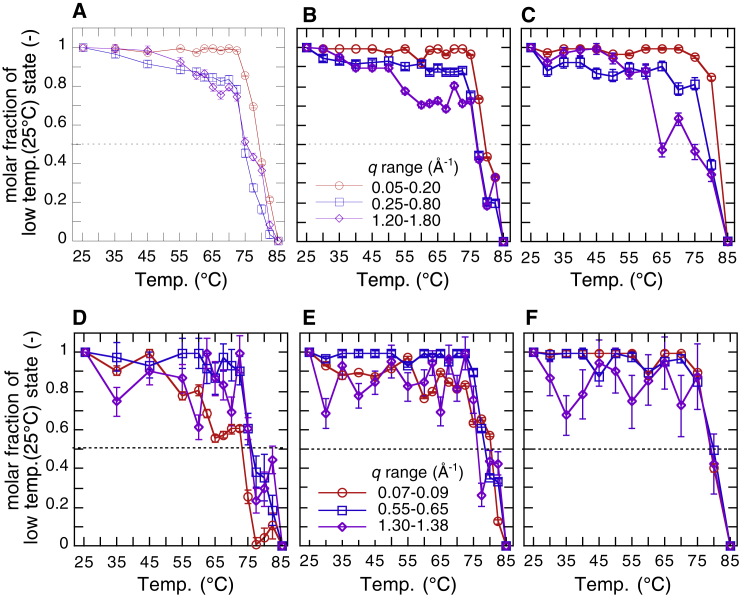
Figure 17.
Molar fraction α (α ≤ 1) of the native structure (at 25°C) of myoglobin at an intermediate temperature in the heating process determined from the scattering curves in different q-ranges by using the TMA analysis. (A) and (D) display data from water solvent, (B) and (E) display data from 10% v/v glycerol solvent, and (C) and (F) display data from 20% v/v glycerol solvent. From (A) to (C), the selected q-ranges are 0.05–0.2 and 0.25–0.8 Å−1, which correspond to the tertiary structure and the internal structure, respectively. From (D) to (F), the selected q-ranges are 0.07–0.09, 0.55–0.65, and 1.30–1.38 Å−1, which correspond to the q-ranges for typical features of amyloid transition, namely oligomerization, pleated sheet stacking, and cross-β structure, respectively. To see this figure in color, go online.