Abstract
Background The epidemiology of ESRD requiring maintenance dialysis (ESRD-D) in large, diverse immigrant populations is unclear.
Methods We estimated ESRD-D prevalence and incidence among immigrants in Ontario, Canada. Adults residing in Ontario in 2014 were categorized as long-term Canadian residents or immigrants according to administrative health and immigration datasets. We determined ESRD-D prevalence among these adults and calculated age-adjusted prevalence ratios (PRs) comparing immigrants to long-term residents. Among those who immigrated to Ontario between 1991 and 2012, age-adjusted ESRD-D incidence was calculated by world region and country of birth, with immigrants from Western nations as the referent group.
Results Among 1,902,394 immigrants and 8,860,283 long-term residents, 1700 (0.09%) and 8909 (0.10%), respectively, presented with ESRD-D. Age-adjusted ESRD-D prevalence was higher among immigrants from sub-Saharan Africa (PR, 2.17; 95% confidence interval [95% CI], 1.84 to 2.57), Latin America and the Caribbean (PR, 2.11; 95% CI, 1.90 to 2.34), South Asia (PR, 1.45; 95% CI, 1.32 to 1.59), and East Asia and the Pacific (PR, 1.34; 95% CI, 1.22 to 1.46). Immigrants from Somalia (PR, 4.18; 95% CI, 3.11 to 5.61), Trinidad and Tobago (PR, 2.88; 95% CI, 2.23 to 3.73), Jamaica (PR, 2.88; 95% CI, 2.40 to 3.44), Sudan (PR, 2.84; 95% CI, 1.53 to 5.27), and Guyana (PR, 2.69; 95% CI, 2.19 to 3.29) had the highest age-adjusted ESRD-D PRs relative to long-term residents. Immigrants from these countries also exhibited higher age-adjusted ESKD-D incidence relative to Western Nations immigrants.
Conclusions Among immigrants in Canada, those from sub-Saharan Africa and the Caribbean have the highest ESRD-D risk. Tailored kidney-protective interventions should be developed for these susceptible populations.
Keywords: hemodialysis, peritoneal dialysis, dialysis, clinical epidemiology, ESRD
Among low- and middle-income countries, noncommunicable diseases such as CKD are increasingly recognized as an important public health concern.1–5 ESRD requiring maintenance dialysis (ESRD-D) affects more than 2.5 million people worldwide and is associated with significant morbidity and mortality, poor quality of life, and greater use of costly health resources.6–11
Canada is a major destination for immigrants from across the globe, especially those from low- and middle-income regions. About 235,000 people immigrate to Canada each year, with about 40% settling in Ontario, Canada’s most populous province. Currently, approximately a third of Ontarians are foreign born.12
Little is known about the epidemiology of ESRD-D with a large and diverse immigrant population. Such information might provide important insights into the genetic and cultural determinants of kidney disease, help optimize preventive strategies and allocation of resources in the destination country, and inform how to support immigrants in a new country.
We conducted this study to estimate the prevalence and incidence rate of ESRD-D among all adult immigrants to Ontario, both by world region and individual country of birth. We estimated the prevalence of ESRD-D to long-term Canadian residents, and further explored the incidence rate of ESRD-D within immigrant groups.
Methods
Design and Setting
We conducted a population-based cross-sectional study in Ontario, Canada, where health care is available for all residents. For newly arrived immigrants, eligibility for universal health care, which includes maintenance dialysis, usually commences shortly after arrival. This study used administrative health datasets that were linked using a unique, encoded identifier. Databases were held at the Institute for Clinical Evaluative Sciences. This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada).
Data Sources
We used seven databases to determine patient baseline and outcome characteristics. The Canadian Organ Replacement Register (CORR) identified patients receiving maintenance dialysis. CORR records are 99% complete for incident dialysis in Ontario.13 We obtained immigration information from the Immigration, Refugees and Citizenship Canada (IRCC)’s Permanent Resident Database. This database contains records for those who landed in Canada after 1985 and captures information at the time of the immigration application. We retrieved demographic and vital status information from the Ontario Registered Persons Database. We used the Ontario Health Insurance Plan Database and the Institute for Clinical Evaluative Sciences Physician Database to obtain information on health claims for inpatient and outpatient physician services. We received diagnostic and procedural information for hospitalizations from the Canadian Institute for Health Information Discharge Abstract Database, and obtained information from emergency room visits from the National Ambulatory Care Reporting System database. Data were complete, with the exception of <0.5% of patients missing data on neighborhood income quintile and rural status. Emigration from Ontario is infrequent, occurring at <0.5% annually.14
Study Cohort
Prevalent Cohort
We included all adults aged 18 years and older, who were alive and residing in Ontario as of April 1, 2014. We categorized individuals as either an “immigrant,” defined as a foreign-born resident of Ontario who arrived in Canada after January 1, 1985, or as “long-term resident,” defined as an Ontario resident who was born in Canada or immigrated to Canada before 1985. Immigrants were defined by a valid birth country code in the IRCC database and we included both refugees (who became permanent residents) and nonrefugees. We further categorized immigrants by country of birth and also by world region of birth (Supplemental Table 1). This grouping, which has been used in previous studies, was chosen because of environmental exposures that might be common in a given region of the world.15–17 To ensure that we included current residents, we excluded those whose date of last contact with the health care system was before March 31, 2011. Those who received a kidney transplant before April 1, 2014 were excluded.
Incident Cohort
To define incident cases of ESRD-D, we created a separate cohort of all adults aged 18 years and older with a valid country code in the IRCC database with a date of immigration between April 1, 1991 and March 31, 2012. The date of cohort entry was defined as 90 days after their immigration date. We excluded individuals with evidence of chronic dialysis or kidney transplantation before their date of cohort entry to remove patients who may have immigrated to Ontario with prevalent ESRD-D.
Events
The primary study event of interest was prevalent ESRD-D, defined as the receipt of maintenance dialysis therapy, defined as any chronic dialysis record in CORR before or on April 1, 2014 (Supplemental Table 2) with no subsequent transplant or dialysis withdrawal observed. The secondary outcome was incident ESRD-D, defined as a new chronic dialysis record in CORR at any time after immigration to Canada.
Statistical Analyses
Participant characteristics were presented as means (SD) for continuous variables and as proportions for categorical variables, and were compared with standardized differences. A standardized difference over 10% is considered to be a meaningful difference.18 We calculated the unadjusted point prevalence (per 100,000 people) and age-adjusted prevalence ratios (PR) and 95% confidence intervals (95% CIs) for ESRD-D, categorized by world region of birth and country of birth (for countries whose immigrants constituted at least six cases of ESRD-D), respectively. For individual country of birth, these metrics were presented for all adults who were born in that country, with further stratification by sex, the presence or absence of diabetes mellitus, and income quintile (for world region). We estimated PRs using log-binomial regression models, adjusted for age.
Among patients with prevalent ESRD-D, we calculated the proportion of patients by immigrant country of birth. These analyses were further stratified by sex, diabetes mellitus status, and dialysis modality. We also compared the characteristics of patients with prevalent ESRD-D by immigration status.
We calculated incidence rate for ESRD-Ds per 1000 person-years categorized by country of birth (for countries whose immigrants constituted at least six cases of ESRD-D) and associated world region. We followed patients until one of the following censoring events: death, preemptive kidney transplantation, or end of follow-up (March 31, 2014). We used Cox proportional hazards regression to calculate age-adjusted hazard ratios and 95% CIs for ESRD-D by world region, compared with individuals born in Western Europe, the United States, Australia, or New Zealand. We collectively referred to these as Western nations, and considered these the referent group in the incidence cohort on the assumption that immigrants from these countries have a similar ethnic composition as native-born Canadians. In an additional analysis where we considered time since immigration, ESRD-D risk by region of immigration was examined in three time intervals: within 5 years of immigration, between 5 and 10 years of immigration, and >10 years of immigration. All analyses were conducted using the statistical analysis system (SAS) software, version 9.4 (SAS Institute, Cary, NC).
Results
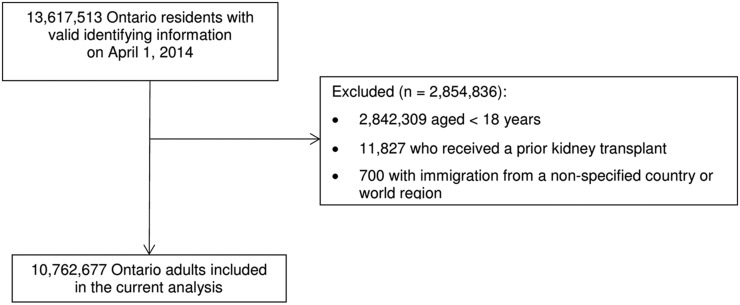
We identified 10,762,972 adult Ontarians, of whom 1,902,394 were immigrants and 8,860,283 were long-term Canadian residents as of April 1, 2014 (Figure 1). Of these, 1700 (0.09%) immigrants and 8909 (0.10%) long-term residents were receiving maintenance dialysis. The leading regions of origin among all immigrants (independent of ESRD-D status) were East Asia and Pacific (26%), South Asia (25%), and Latin America and the Caribbean (14%). As compared with long-term Canadian residents, immigrants were younger, less likely to reside in a rural area, and a larger proportion were in the lowest income quintile (Supplemental Table 3). The point prevalence values for ESRD-D among immigrants and long-term Canadian residents were 89.4 per 100,000 and 100.5 per 100,000, respectively. After adjusting for age, the prevalence of ESRD-D was higher among immigrants compared with long-term Canadian residents (age-adjusted PR, 1.29’ 95% CI, 1.23 to 1.26; long-term Canadian residents are the referent group). Comparisons of immigrants with and without ESRD-D are shown in Supplemental Table 4.
Figure 1.
Flow diagram demonstrating creation of the final study cohort.
Characteristics of Prevalent ESRD-D Patients, by Immigrant Status
Immigrants with prevalent ESRD-D were younger than long-term residents with ESRD-D (mean 62.4 versus 67.0 years, SD 31%), more likely to have GN as the cause of ESRD-D (17.5% versus 12.8%, SD 13%), reside in neighborhoods in the lowest income quintile (35.8% versus 24.8%, SD 24%), less likely to reside in a rural area (0.5% versus 13.5%, SD 53%), and had fewer prior primary care visits (8.2 versus 11.0 visits, SD 18%) or hospitalizations (0.9 versus 1.1, SD 17%) in the year preceding April 1, 2014. Immigrants were more likely to receive peritoneal dialysis (21.2% versus 16.6%, SD 0.12) (Table 1).
Table 1.
Characteristics of Ontario adults with ESRD requiring maintenance dialysis as of April 1, 2014, categorized as immigrants or long-term Canadian residents
| Characteristic | Immigrants, n=1700 | Long-Term Resident, n=8909 | Standardized Difference, % |
|---|---|---|---|
| Demographics | |||
| Age, yr (mean±SD) | 62.4±15.3 | 67.0±14.6 | 31 |
| 18–29 | 44 (2.6) | 151 (1.7) | 6 |
| 30–39 | 100 (5.9) | 280 (3.1) | 14 |
| 40–49 | 199 (11.7) | 678 (7.6) | 14 |
| 50–59 | 374 (22.0) | 1341 (15.1) | 18 |
| 60–69 | 380 (22.4) | 2220 (24.9) | 6 |
| 70–79 | 362 (21.3) | 2308 (25.9) | 11 |
| 80–89 | 215 (12.6) | 1732 (19.4) | 19 |
| ≥90 | 26 (1.5) | 199 (2.2) | 5 |
| Women | 751 (44.2) | 3591 (40.3) | 8 |
| Income quintile | |||
| First (lowest) | 609 (35.8) | 2207 (24.8) | 24 |
| Second | 404 (23.8) | 1964 (22.0) | 4 |
| Third | 322 (19.0) | 1783 (20.1) | 3 |
| Fourth | 223 (13.1) | 1596 (17.9) | 13 |
| Fifth (highest) | 142 (8.4) | 1359 (15.3) | 21 |
| Rural residencea | 9 (0.5) | 1206 (13.5) | 53 |
| Dialysis characteristics | |||
| Dialysis vintage (mean±SD) | 3.7±3.4 | 3.5±3.5 | 6 |
| Dialysis modality | |||
| Conventional hemodialysis | 1270 (74.7) | 7066 (79.3) | 12 |
| Home hemodialysis | 72 (4.2) | 362 (4.1) | 3 |
| Peritoneal dialysis | 358 (21.1) | 1481 (16.6) | 12 |
| Dialysis access | |||
| Catheter | 428 (25.2) | 2425 (27.2) | 5 |
| Fistula/graft | 93 (5.5) | 535 (6.0) | 2 |
| Unknown | 130 (7.6) | 460 (5.2) | 10 |
| Missing | 1049 (61.7) | 5489 (61.6) | 0 |
| Cause of ESRD | |||
| GN | 299 (17.6) | 1137 (12.8) | 13 |
| Cystic kidney disease | 55 (3.2) | 390 (4.4) | 6 |
| Diabetes | 663 (39.0) | 3340 (37.5) | 3 |
| Renal vascular | 290 (17.1) | 1462 (16.4) | 2 |
| Other | 208 (12.2) | 1394 (15.6) | 10 |
| Unknown/missing | 185 (10.9) | 1186 (13.3) | 7 |
| Comorbidities | |||
| Diabetes (in past 10 yr) | 1111 (65.4) | 5865 (65.8) | 1 |
| Myocardial infarction (in past 2 yr) | 123 (7.2) | 744 (8.4) | 4 |
| Stroke (in past 2 yr) | 38 (2.2) | 274 (3.1) | 6 |
| Hypertension (in past 2 yr) | 1157 (68.1) | 5896 (66.2) | 4 |
| Health care utilization (in past 1 yr) | |||
| Previous visits to primary care provider (mean±SD) | 8.2±13.1 | 11.0±16.7 | 18 |
| 0 | 278 (16.4) | 1077 (12.1) | 12 |
| 1–2 | 344 (20.2) | 1590 (17.8) | 6 |
| 3–4 | 266 (15.6) | 1244 (14.0) | 5 |
| 5+ | 812 (47.8) | 4998 (56.1) | 17 |
| Previous hospitalizations (mean±SD) | 0.9±1.3 | 1.1±1.6 | 17 |
| 0 | 944 (55.5) | 4310 (48.4) | 14 |
| 1 | 402 (23.6) | 2144 (24.1) | 1 |
| 2 | 164 (9.6) | 1164 (13.1) | 11 |
| 3+ | 190 (11.2) | 1291 (14.5) | 10 |
All data are presented as a number (%) unless otherwise stated.
Refers to location of residence with a population size <10,000 persons.
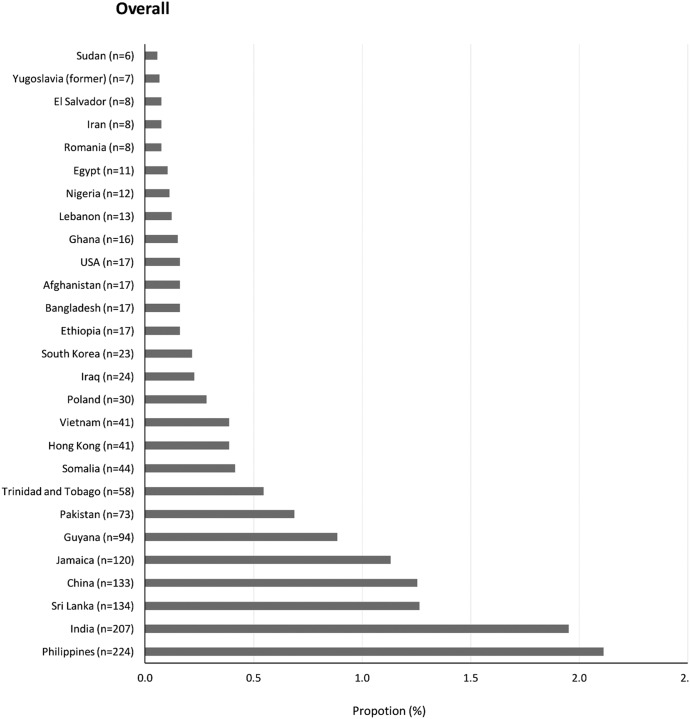
Among patients with ESRD-D, the countries with the highest proportion of immigrants on maintenance dialysis were the Philippines, India, Sri Lanka, China, and Jamaica (Figure 2). A similar proportion was seen for patients stratified by sex (Supplemental Figure 1), diabetes mellitus (Supplemental Figure 2), and dialysis modality (Supplemental Figure 3).
Figure 2.
The proportion of immigrants with ESRD treated with maintenance dialysis by country of birth. Immigrants from the Philippines, India, Sri Lanka, and China represented the largest proportion of immigrants with ESRD-D.
Maintenance Dialysis Prevalence by Region and Country of Birth
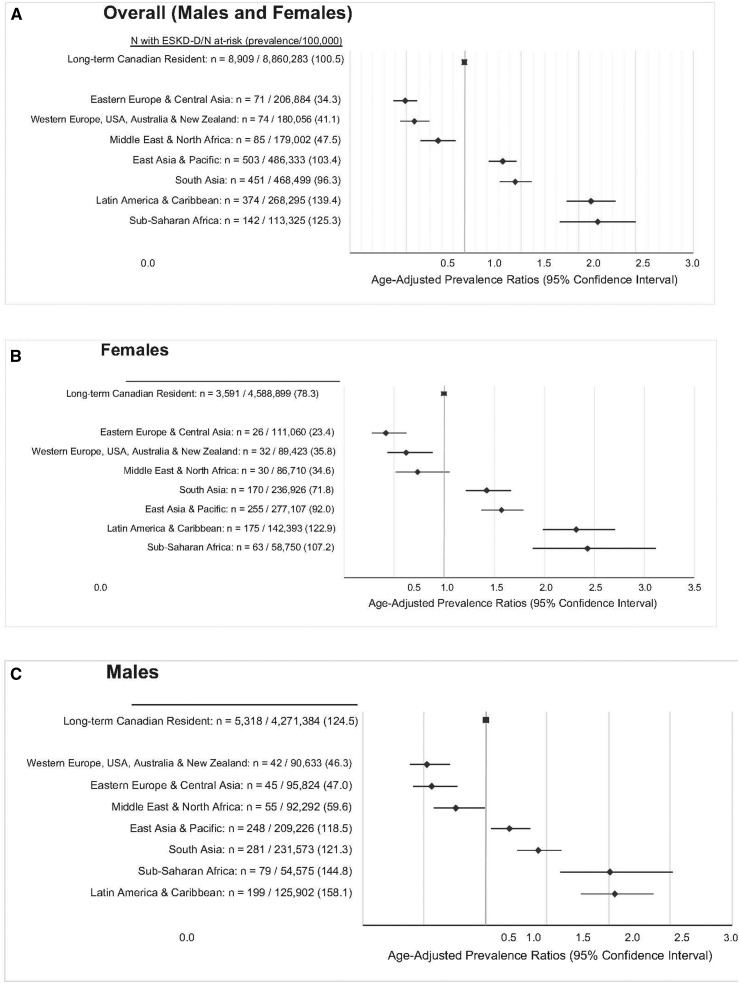
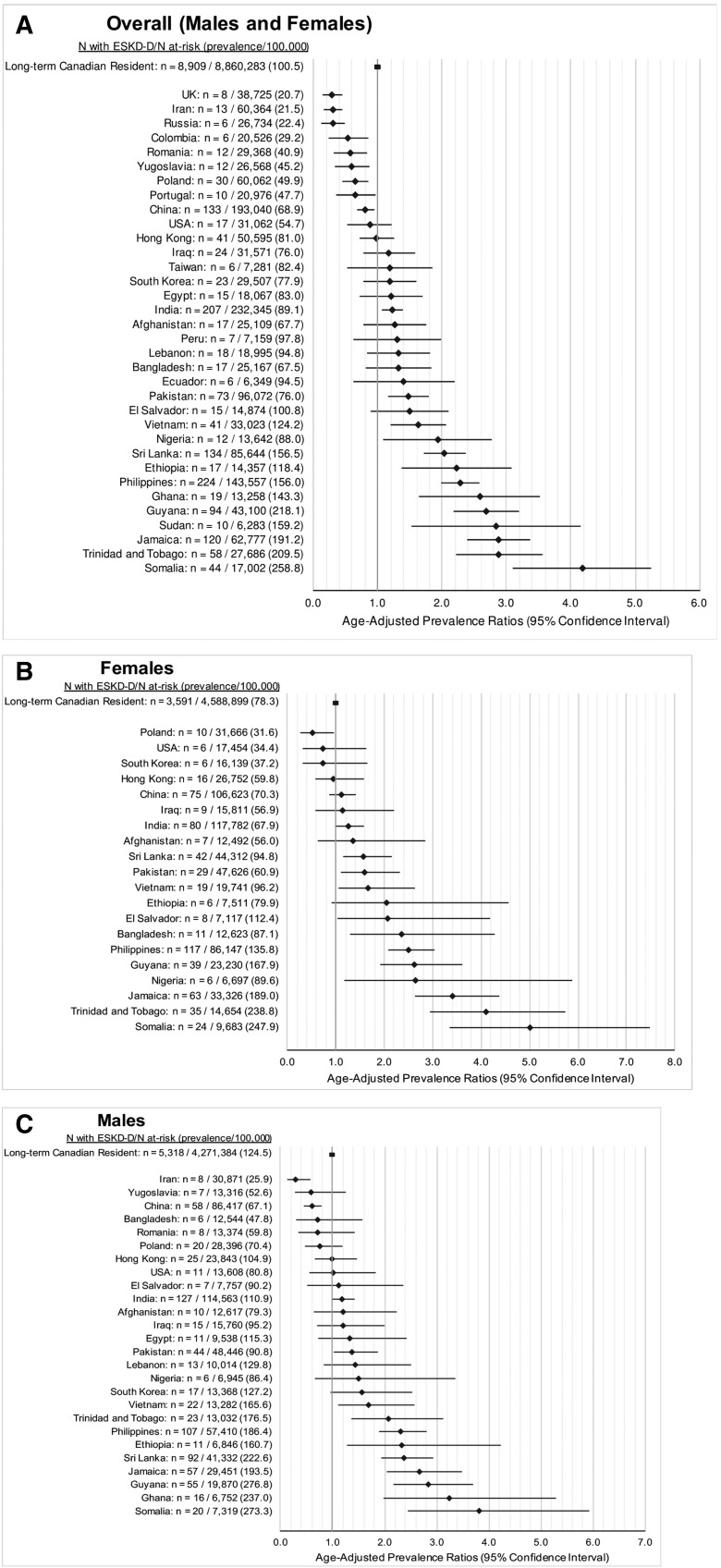
The age-adjusted prevalence of ESRD-D was higher among those from sub-Saharan Africa (PR, 2.17; 95% CI, 1.84 to 2.57), Latin America and the Caribbean (PR, 2.11; 95% CI, 1.90 to 2.34), South Asia (PR, 1.45; 95% CI, 1.32 to 1.59), and East Asia and the Pacific (PR, 1.34; 95% CI, 1.22 to 1.46) (Figure 3). Immigrants born in Somalia (PR, 4.18; 95% CI, 3.11 to 5.61), Trinidad and Tobago (PR, 2.88; 95% CI, 2.23 to 3.73), Jamaica (PR, 2.88; 95% CI, 2.40 to 3.44), Sudan (PR, 2.84; 95% CI, 1.53 to 5.27), and Guyana (PR, 2.69; 95% CI, 2.19 to 3.29) in addition to the countries listed in Figure 4 had a higher relative prevalence of ESRD-D compared with long-term Canadian residents. The prevalence of ESRD-D was much higher in those with versus without diabetes mellitus, but the pattern by country of origin was similar to that given above (Supplemental Figure 4). Similarly, an analysis stratified by income quintile did not reveal significant differences in age-adjusted PR by world region across income quintiles (P=0.32; Supplemental Table 5).
Figure 3.
Age-adjusted PRs of ESRD requiring maintenance dialysis, by region of immigration versus long-term Canadian residents. All adults (A) and by sex (B and C). In both men and women, immigrants from the world regions of Latin America and The Caribbean, Sub-Saharan Africa, South Asia, and East Asia and Pacific had a higher prevalence of ESRD requiring maintenance dialysis compared to long-term residents.
Figure 4.
Age-adjusted PRs of ESRD requiring maintenance dialysis of long-term Canadian residents and immigrants by country of birth. All adults (A) and by sex (B and C). Only countries with six or more patients with ESRD requiring maintenance dialysis were reported for ICES privacy reasons. Overall, the prevalence of ESRD requiring maintenance dialysis was highest in those immigrating from Somalia, Trinidad and Tobago, Jamaica and Sudan compared to long term residents.
Maintenance Dialysis Incidence Rate by Region and Country of Birth
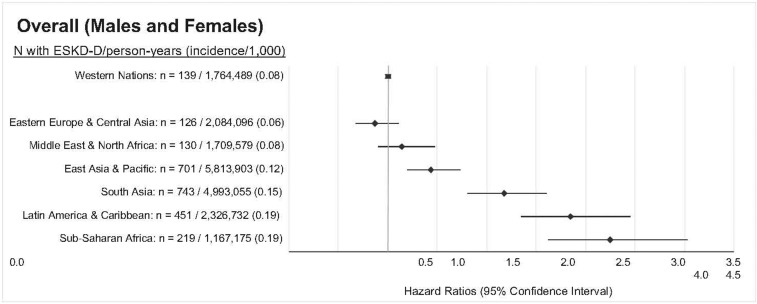
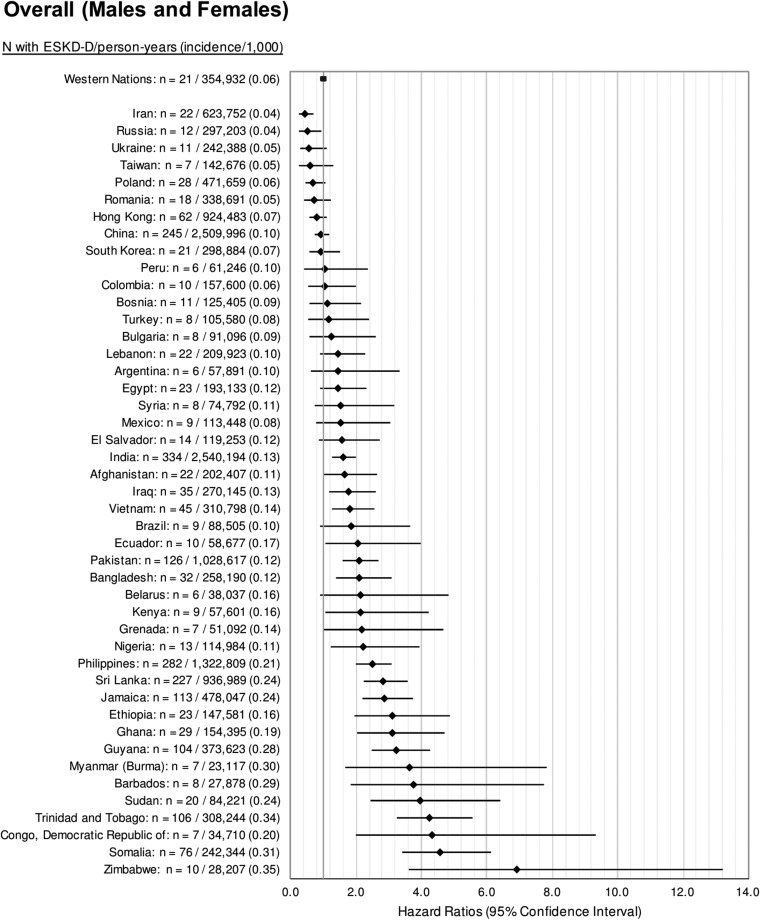
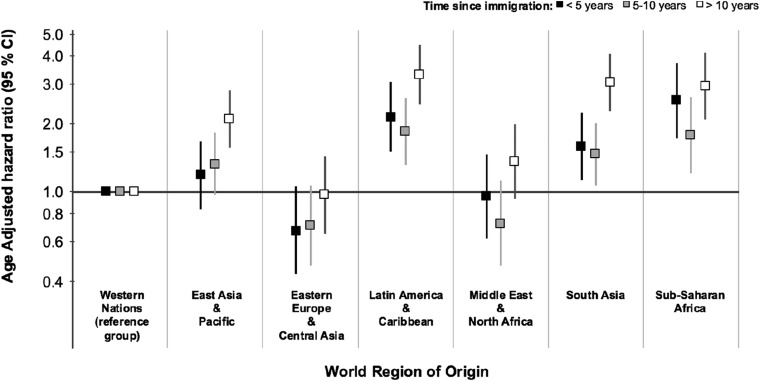
We studied the incidence of ESRD-D in 1,686,407 immigrants to Ontario who arrived between 1991 and 2012. A total of 2509 immigrants developed ESRD-D over the study period. The median time to ESRD-D among all immigrant groups was 9.0 years (IQR, 4.7–14.1 years). Censoring events included preemptive transplantation (n=305) and death (n=36,475), and did not significantly differ by world region (Supplemental Table 6). Immigrants from sub-Saharan Africa, Latin America, South Asia, and East Asia and Pacific had a significantly higher incidence rate of ESRD-D compared with immigrants from Western Europe, the United States, Australia, and New Zealand (Western nations) (Figure 5). Results stratified by sex and diabetes are presented in Supplemental Table 7. Immigrants from Zimbabwe, Kenya, Democratic Republic of Congo, India, Bangladesh, Iraq, Myanmar (Burma), Afghanistan, Barbados, Grenada, and Ecuador had higher incidence rates compared with immigrants from Western nations (Figure 6). Examining the age-adjusted hazard ratios of ESRD-D within 5 years of immigration, 5 to ≤10 years since immigration, and >10 years since immigration, we observed a slighter higher age-adjusted hazard ratio for ESRD-D after 10 years of immigration, particularly for those from East Asia, South Asia, and Latin America and the Caribbean (Figure 7).
Figure 5.
Age-adjusted hazard ratios for the incidence rate of ESRD requiring maintenance dialysis by region of immigration. Immigrants from Sub-Saharan Africa, Latin America and Caribbean, South Asia, and East Asia and Pacific had a higher incidence of ESRD-D compared to immigrants from Western nations (immigrants from Western Europe, the United States, Australia, and New Zealand), the referent group.
Figure 6.
Age-adjusted hazard ratios for the incidence rate of ESRD requiring maintenance dialysis by country of birth. The highest incidence of ESRD-D occurred in immigrants from Zimbabwe, Somalia, Congo, Democratic Republic of, Trinidad and Tobago compared to immigrants from Western nations (Western Europe, the United States, Australia, and New Zealand), the referent group. Only countries with six or more patients with ESRD requiring maintenance dialysis were reported for ICES privacy reasons.
Figure 7.
Age-adjusted hazard ratios for the incidence rate of ESRD requiring maintenance dialysis by world region and time since immigration. A slightly higher age-adjusted hazard ratio for ESRD-D after 10 years of immigration was seen, particularly for those from East Asia, South Asia, and Latin America and The Caribbean compared to Western nations which comprise immigrants from Western Europe, the United States, Australia, and New Zealand.
Discussion
Using unique population-based linked administrative datasets, within a province where nearly one third of residents are immigrants, we observed that immigrants born in Somalia, Trinidad and Tobago, Jamaica, Sudan, Guyana, Ghana, the Philippines, Ethiopia, Sri Lanka, and Nigeria had an especially high prevalence of ESRD-D compared with long-term residents. Patients who were born in the Philippines, India, China, Sri Lanka, and Jamaica represented the largest proportion of immigrant adults currently receiving maintenance dialysis.
These novel data describe the prevalence of ESRD-D among immigrants, in a setting where dialysis is universally available within a single provincial health care system. Prior studies, largely conducted in the United States, did not assess immigrant status, but focused on inter-racial differences in kidney disease.19–21 A four-fold higher risk of ESRD was previously seen among blacks compared with whites, in addition to a higher but less pronounced risk among Hispanic Americans and Asian Americans.21 Although they represent 13% of the United States population, blacks constitute >35% of all Americans who receive dialysis.22 What remains unclear is the contribution of socioeconomic factors and differences in access to care that may explain this phenomenon in the United States. We found that immigrants from African and African Caribbean countries had the highest age-adjusted prevalence of ESRD-D. It is interesting to note that among immigrants, there were no appreciable differences in the frequency of predialysis nephrology visits in the 90 and 365 days before dialysis initiation by world region of immigration (Supplemental Table 8). It is therefore unlikely that differential access to health care was a major driver of the results that we observed. Another recent study observed a prevalence of CKD of 14% within sub-Saharan Africa.23 In part, our findings and those of others may reflect the higher prevalence of diabetes, hypertension, chronic hepatitis C, and other risk factors among residents of these countries who subsequently immigrate to Canada.15,24 Although diabetes is more common among those from the Caribbean and sub-Saharan Africa, the prevalence of ESRD-D was also relatively higher in nondiabetic immigrants from those regions.15,16
In nondiabetic adults, the prevalence of ESRD-D seen was highest among those from Ghana. In one study of 712 patients with hypertension in Ghana, 47% had some degree of CKD.25 This population may have variants in the APOLI gene, which were associated with higher rates of ESRD and CKD progression among blacks, independent of diabetes status.26 Hypertension and diabetes, in conjunction with novel genetic factors, might explain the heightened susceptibility to ESRD-D among certain African subpopulations that immigrate to Canada.
We observed a higher incidence rate and prevalence of ESRD-D among immigrants from the Philippines. In absolute terms, this group represents the largest number of immigrants currently receiving dialysis in Ontario, Canada (Figure 2) and parallels the steep rise in ESRD-D incidence in the Philippines in the last 15 years.27 Previous insights into kidney disease risk among these individuals have largely stemmed from studies among Filipino patients receiving dialysis in Hawaii revealing a two-fold higher rate of GN compared with those receiving dialysis in the continental United States.28 This finding may be related to the overall higher rate of IgA nephropathy among Asians. In this study, we observed a higher prevalence of both diabetic and nondiabetic ESRD-D among immigrants from the Philippines, which is also consistent with previous studies suggesting that Filipino Americans also have a high prevalence of diabetes.29
Preeclampsia is an established risk factor for ESRD-D years after pregnancy.30,31 In a recent study, we showed that the risk of preterm preeclampsia was highest in women from Nigeria (6.6 per 1000), the Philippines (7.2 per 1000), Colombia (7.5 per 1000), Jamaica (7.9 per 1000), and Ghana (8.3 per 1000)—data that largely parallels what we have shown herein for ESRD-D.32 Preeclampsia and ESRD-D may share some common pathophysiologic pathways beyond chronic hypertension and diabetes. Although higher circulating levels of soluble fms-like tyrosine kinase-1 lead to endothelial dysfunction and the evolution of preeclampsia, studies are lacking about the long-term persistence of soluble fms-like tyrosine kinase-1 after preeclampsia, and the evolution of kidney disease.33,34
The overall prevalence of ESRD-D was lower for immigrants from Western countries, the Middle East, and North Africa compared with long-term Canadian residents. This may be partly explained by the healthy immigrant effect of these groups compared with the long-term Canadian population.35,36 It is likely that the rigorous health screening of prospective immigrants leads to their under-representation in the ranks of ESRD-D. However, among countries in which we saw a higher incidence rate and prevalence of ESRD-D, it is possible that genetic and environmental factors may outweigh the healthy immigrant effect. It is interesting to note that after 10 years of immigration, the age-adjusted ESRD-D risk was higher among all regions, relative to Western nations, compared with that seen within 5 years of immigration. These findings may relate to the acquisition of deleterious lifestyle habits, including patterns of physical activity and dietary habits since immigration, that have been associated with additional adverse health outcomes including obesity, hypertension, and cardiovascular disease.37
This is the first study to rigorously examine the relationship between immigration and CKD. We used a rich immigration dataset that allowed us to accurately examine the risk of ESRD-D by specific country of birth. Although we did not study the entire spectrum of CKD, we focused on its most extreme, clinically significant, and most costly manifestation, ESRD-D. As compared with nondialysis requiring CKD, ESRD-D is accurately recorded in CORR, a national registry that captures >99% of maintenance dialysis and solid-organ transplant recipient activity in Canada.13,38 Finally, the study was conducted in a jurisdiction with single payer universal health coverage, thereby ensuring that our findings are complete and encompass the entirety of the population, including immigrants who received health coverage upon arrival in Canada.
Our study has evident limitations. A key weakness is, for the long-term Canadian residents cohort, the inability to distinguish between individuals who were born in Canada and long-term residents who immigrated to Canada before 1985. Such misclassification may have attenuated the magnitude of the relative relationship between immigrant status and the risk of ESRD-D. We had no information on the ethnicity of long-term residents, some of whom themselves might have been immigrants. We partially accounted for this in our additional analysis that considered ESRD-D risk by the time since immigration. Finally, given that we did not prospectively follow individuals with late-stage CKD, we could not explore whether our findings may have related to differential receipt of dialysis versus conservative care among immigrant subgroups. We also had no way of excluding those who were transplanted in their country of origin before arrival to Canada. These individuals may have been at risk for graft failure and dialysis initiation, which would have been included in our outcome. However, kidney transplantation would have been unlikely to occur with high frequency among the low- and middle-income countries in which we observed a higher prevalence of ESRD-D.39,40 Moreover, our point prevalence definition of ESRD-D excluded kidney transplant recipients. Previous studies found that certain ethnic groups may have lower rates of kidney transplantation, which may have affected our prevalent rates of ESRD-D.41,42 We addressed this limitation by the inclusion of an analysis of the risk of ESRD-D among an incident cohort of immigrants where the preemptive transplantation rate was extremely low (n=305, 0.02%) and did not appreciably affect our hazard ratios for ESRD-D incidence among immigrant groups.
We showed that the prevalence and incidence rate of ESRD-D differs significantly by world region and country of birth. Such information may help to inform strategies aimed at prevention of kidney disease among immigrants originating from countries at high risk for ESRD-D, and help address the burden of ESRD-D by promoting living kidney donation among these groups. Lastly, these findings may inform the need for expanded screening procedures for kidney disease for immigrants originating from high-risk countries.
Disclosures
J.P. has received speaking honoraria from Baxter Healthcare and Davita Healthcare Partners, and consulting fees from Baxter Healthcare, Fresenius Medical Care, Otsuka, Janssen Ortho Shire, Takeda, and Boehringer–Ingelheim, as well as unrestricted research support from Baxter Healthcare and salary support from Arbor Research Collaborative for Health. R.W. has received speaking fees and unrestricted research support from Baxter Healthcare.
Supplementary Material
Acknowledgments
J.P., E.M., D.M.N., A.X.G., J.G.R., and R.W. made substantial contributions to the study design. J.P., A.X.G., A.H.L., M.M.S., Z.H., J.G.R., and R.W. played significant roles in drafting and revising the manuscript. V.S.T. made the figures. All authors approve the final version of the manuscript.
A.X.G. is supported by a Clinician Investigator Award from the Canadian Institute for Health Research (CIHR) and the Dr. Adam Linton Chair in Kidney Health Analytics. J.G.R. holds a CIHR Applied Health Research Chair. M.M.S. is supported by the Jindal Research Chair for the Prevention of Kidney Disease. A.L. is supported by a CIHR Fellowship. This study was conducted by the Institute for Clinical Evaluative Sciences (ICES) Western Site through the ICES Kidney, Dialysis and Transplantation Research Program. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. ICES Western is funded by an operating grant from the Academic Medical Organization of Southwestern Ontario.
No endorsement by ICES, the Academic Medical Organization of Southwestern Ontario, or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. Parts of this material are on the basis of data and/or information compiled and provided by Canadian Institutes of Health Research. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of Canadian Institutes of Health Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Greater Burden of ESRD among Immigrants: Kwa nini?,” on pages 1789–1790.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017101055/-/DCSupplemental.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al.: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al.: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Stanifer JW, Muiru A, Jafar TH, Patel UD: Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant 31: 868–874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization : Global status report on noncommunicable diseases, Geneva, Switzerland, World Health Organization, 2010 [Google Scholar]
- 6.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al.: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 7.van Walraven C, Manuel DG, Knoll G: Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 63: 491–499, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Evans RW, Manninen DL, Garrison LP Jr, Hart LG, Blagg CR, Gutman RA, et al.: The quality of life of patients with end-stage renal disease. N Engl J Med 312: 553–559, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Tentori F, Mapes DL: Health-related quality of life and depression among participants in the DOPPS: Predictors and associations with clinical outcomes. Semin Dial 23: 14–16, 2010 [DOI] [PubMed] [Google Scholar]
- 10.De Vecchi AF, Dratwa M, Wiedemann ME: Healthcare systems and end-stage renal disease (ESRD) therapies--an international review: Costs and reimbursement/funding of ESRD therapies. Nephrol Dial Transplant 14[Suppl 6]: 31–41, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, et al.: Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis 40: 611–622, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Statistics Canada : 150 years of immigration in Canada, 2016. Available at https://www.fin.gov.on.ca/en/economy/demographics/census/cenhi16-8.html. Accessed April 18, 2018 [Google Scholar]
- 13.Data Quality Documentation for Users : Canadian Organ Replacement Register, 2004 to 2013 Data, Ottawa, ON, Canadian Institute for Health Information, 2015 [Google Scholar]
- 14.Ontario population projections update 2012-2036, Toronto, Canada, Ontario Ministry of Finance: Government of Ontario; Available at https://www.fin.gov.on.ca/en/economy/demographics/projections/#s4f. Accessed April 18, 2018 [Google Scholar]
- 15.Creatore MI, Booth GL, Manuel DG, Moineddin R, Glazier RH: Diabetes screening among immigrants: A population-based urban cohort study. Diabetes Care 35: 754–761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creatore MI, Moineddin R, Booth G, Manuel DH, DesMeules M, McDermott S, et al.: Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ 182: 781–789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urquia ML, Ying I, Glazier RH, Berger H, De Souza LR, Ray JG: Serious preeclampsia among different immigrant groups. J Obstet Gynaecol Can 34: 348–352, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simulat 38: 1228–1234, 2009 [Google Scholar]
- 19.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM: White/black racial differences in risk of end-stage renal disease and death. Am J Med 122: 672–678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ: Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lipworth L, Mumma MT, Cavanaugh KL, Edwards TL, Ikizler TA, Tarone RE, et al.: Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS One 7: e48407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Renal Data System : USRDS 2017 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 23.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al.: The epidemiology of chronic kidney disease in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health 2: e174–e181, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Greenaway C, Azoulay L, Allard R, Cox J, Tran VA, Abou Chakra CN, Steele R, Klein M: A population-based study of chronic hepatitis C in immigrants and non-immigrants in Quebec, Canada. BMC Infect Dis 17: 140, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osafo C, Mate-Kole M, Affram K, Adu D: Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail 33: 388–392, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al.; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute of Diabetes and Digestive and Kidney Diseases; USRDS Coordinating Center : U.S. renal data system annual data report, researcher’s guide, reference tables, ADR slides, Ann Arbor, MI, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 28.Norris KC, Agodoa LY: Unraveling the racial disparities associated with kidney disease. Kidney Int 68: 914–924, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Cuasay LC, Lee ES, Orlander PP, Steffen-Batey L, Hanis CL: Prevalence and determinants of type 2 diabetes among Filipino-Americans in the Houston, Texas metropolitan statistical area. Diabetes Care 24: 2054–2058, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Wang IK, Muo CH, Chang YC, Liang CC, Chang CT, Lin SY, et al.: Association between hypertensive disorders during pregnancy and end-stage renal disease: A population-based study. CMAJ 185: 207–213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Ray JG, Wanigaratne S, Park AL, Bartsch E, Dzakpasu S, Urquia ML: Preterm preeclampsia in relation to country of birth. J Perinatol 36: 718–722, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al.: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luft FC: Soluble fms-like tyrosine kinase-1 and atherosclerosis in chronic kidney disease. Kidney Int 85: 238–240, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Gushulak BD, Pottie K, Hatcher Roberts J, Torres S, DesMeules M; Canadian Collaboration for Immigrant and Refugee Health : Migration and health in Canada: Health in the global village. CMAJ 183: E952–E958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vang ZM, Sigouin J, Flenon A, Gagnon A: Are immigrants healthier than native-born Canadians? A systematic review of the healthy immigrant effect in Canada. Ethn Health 22: 209–241, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Sanou D, O’Reilly E, Ngnie-Teta I, Batal M, Mondain N, Andrew C, et al.: Acculturation and nutritional health of immigrants in Canada: A scoping review. J Immigr Minor Health 16: 24–34, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moist LM, Richards HA, Miskulin D, Lok CE, Yeates K, Garg AX, et al.: A validation study of the Canadian Organ Replacement Register. Clin J Am Soc Nephrol 6: 813–818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bello AK, Alrukhaimi M, Ashuntantang GE, Bellorin-Font E, Gharbi MB, Braam B, et al. : Global overview of health systems oversight and financing for kidney care. Kidney Int Suppl 8: 41–51, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, et al. : Global kidney health atlas: A report by the International Society of Nephrology on the current state of organization and structures for kidney care across the globe, Brussels, Belgium International Society of Nephrology, 2017 [Google Scholar]
- 41.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, et al. : Racial disparities in access to renal transplantation–clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeates KE, Schaubel DE, Cass A, Sequist TD, Ayanian JZ: Access to renal transplantation for minority patients with ESRD in Canada. Am J Kidney Dis 44: 1083–1089, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.