Figure 2.
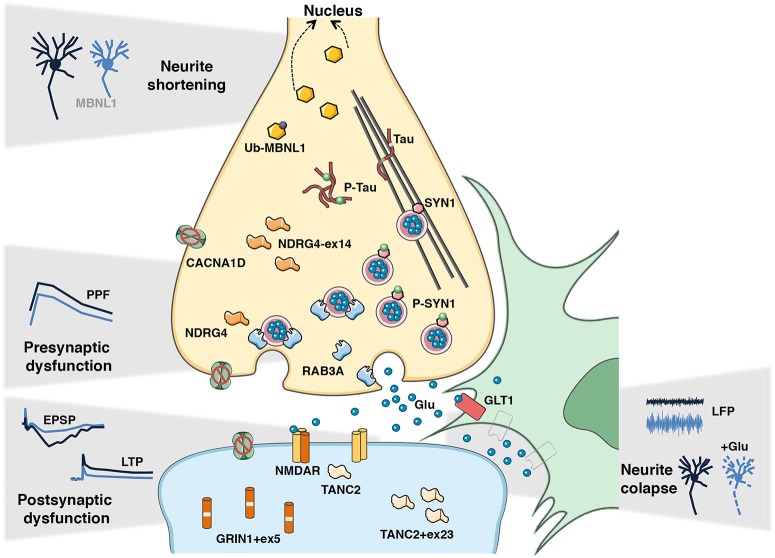
Candidate disease intermediates of DM synaptic dysfunction and learning deficits. The dysregulated disease mechanisms in the CNS of DM1 mouse models appear to involve both pre- and postsynaptic events, which lead to global synaptic dysfunction and consequent cognitive and memory deficits. In the pre-synaptic compartment the hyperphosphorylation of SYN1 and upregulation of RAB3A, together with the missplicing of Mapt/Tau, Ndrg4, and Cacna1d may contribute to impaired short-term synaptic plasticity, notably through decreased paired-pulse facilitation (PPF) detected in DMSXL mice. In the postsynaptic counterpart, the missplicing of Grin1, Tanc2, and Cacna1d may disrupt the functioning of the voltage-gated NMDA receptor, and consequently NMDAR-mediated mechanisms of long-term potentiation (LTP) detected in Mbnl2 KO and EpA960 mice. Reduced GLT1 levels in neighboring astrocytes likely result in neuronal hyperexcitability, demonstrated by increased local field potentiation (LFP) in DMSXL mice, and it can ultimately lead to neuronal damage and neurite collapse in the presence of excessive glutamate. The mislocalization of MBNL1 into the nucleus following abnormal de-ubiquitination decreases neuritogenesis and affects neuronal morphology in EpA960 mice. Together these events likely mediate defective synaptic transmission and abnormal brain connectivity behind DM cognitive and behavioral changes.