Figure 3.
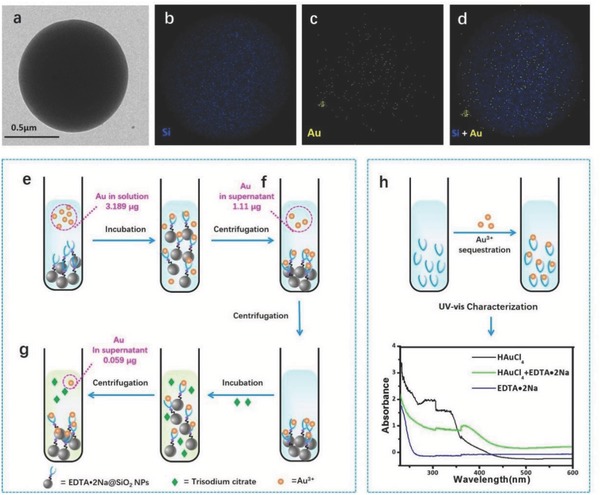
Elemental distribution analysis of EDTA•2Na@SiO2‐Au chelates and investigation of the efficacy of EDTA•2Na on sequestering Au3+ ions by ICP‐MS technique. a) TEM image of EDTA•2Na@SiO2‐Au chelates. b–d) EDX mapping image of EDTA•2Na@SiO2‐Au chelates (blue = Si, yellow = Au). 500 µL of HAuCl4 (2 × 10−3 m) were added to EDTA•2Na@SiO2 solutions for chelation and detected through X‐ray (EDX) mapping. e,f) 500 µL of HAuCl4 (0.00638 g L−1) were added to EDTA•2Na@SiO2 solutions for chelation and after centrifugation the changes in Au contents were detected through ICP‐MS. g) The precipitates of EDTA•2Na@SiO2‐Au chelates obtained from (b) were redispersed in water and further incubated with 500 µL of trisodium citrate solution (2 × 10−3 m). The contents of Au in supernatant after centrifugation were detected through ICP‐MS. h) UV–vis spectra of HAuCl4, EDTA•2Na, and their mixture.
