Figure 3.
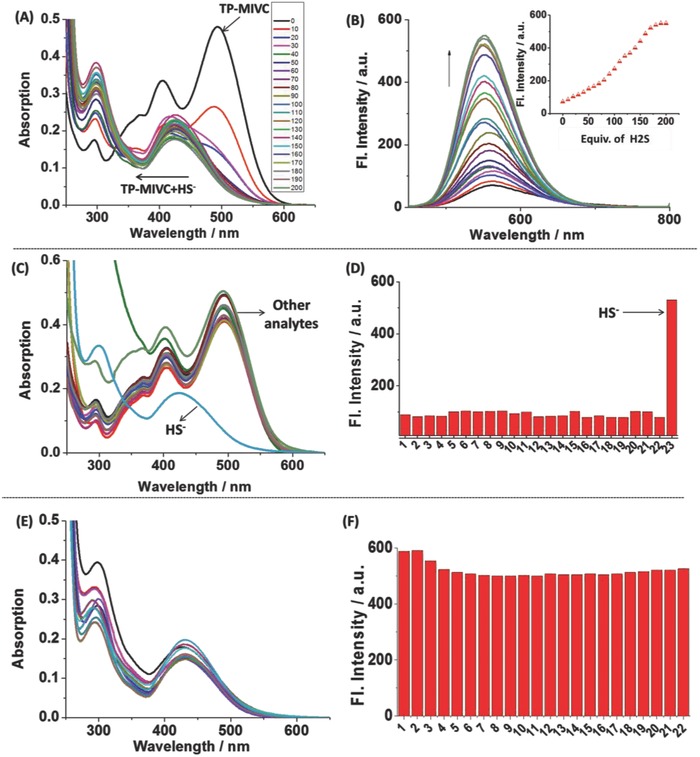
A) Ultraviolet titration experiment of TP‐MIVC (10 × 10−6 m) in pH 7.4 PBS buffer solution with the addition of H2S (0–200 equiv.). B) Fluorescence titration experiment of TP‐MIVC (10 × 10−6 m) in buffer solution with increasing of H2S (0–200 equiv.) at room temperature. Inset: Trend of fluorescence changes at 550 nm of TP‐MIVC (10 × 10−6 m) with increasing of H2S. C) Absorption spectra of TP‐MIVC (10 × 10−6 m) in the presence of various relevant analytes at room temperature. D) Fluorescence responses of TP‐MIVC (10 × 10−6 m) in the presence of various relevant analytes. Legend: 1, TP‐MIVC; 2, Br−; 3, Ca2+; 4, Cl−; 5, Cu2+; 6, Cys; 7, GSH; 8, H2O2; 9,Hcy; 10, HSO3 − 11, I−; 12, K+; 13, Mg2+; 14, Na+; 15, NO2 −; 16, NO3 −; 17, PO4 2−; 18, S2−; 19, S2O3 2−; 20, SO3 2−; 21, SO4 2−; 22, Zn2+; and 23, HS−. λex: 405 nm. E) Absorption spectra of TP‐MIVC (10 × 10−6 m) in pH 7.4 PBS buffer to HS− in the presence of various analytes at room temperature. F) Fluorescence responses of TP‐MIVC (10 × 10−6 m) in pH 7.4 PBS buffer to HS− in the presence of various analytes at room temperature. 1, TP‐MIVC‐HS−; 2, TP‐MIVC‐HS− ‐Br−; 3, TP‐MIVC‐HS− ‐Ca2+; 4, TP‐MIVC‐HS− ‐Cl−; 5, TP‐MIVC‐HS− ‐Cu2+; 6, TP‐MIVC‐HS− ‐Cys; 7, TP‐MIVC‐HS− ‐GSH; 8, TP‐MIVC‐HS− ‐H2O2; 9, TP‐MIVC‐HS− ‐Hcy; 10, TP‐MIVC‐HS− ‐HSO3 −; 11, TP‐MIVC‐HS− ‐I−; 12, TP‐MIVC‐HS− ‐K+; 13, TP‐MIVC‐HS− ‐Mg2+; 14, TP‐MIVC‐HS− ‐Na+; 15, TP‐MIVC‐HS− ‐NO2 −; 16, TP‐MIVC‐HS− ‐NO3 −; 17, TP‐MIVC‐HS− ‐PO4 2−; 18, TP‐MIVC‐HS− ‐S2−; 19, TP‐MIVC‐HS− ‐S2O3 2−; 20, TP‐MIVC‐HS− ‐SO3 2−; 21, TP‐MIVC‐HS− ‐SO4 2−; 22, TP‐MIVC‐HS− ‐Zn2+.
