Fig. 5. Structural determinants of HD5 function.
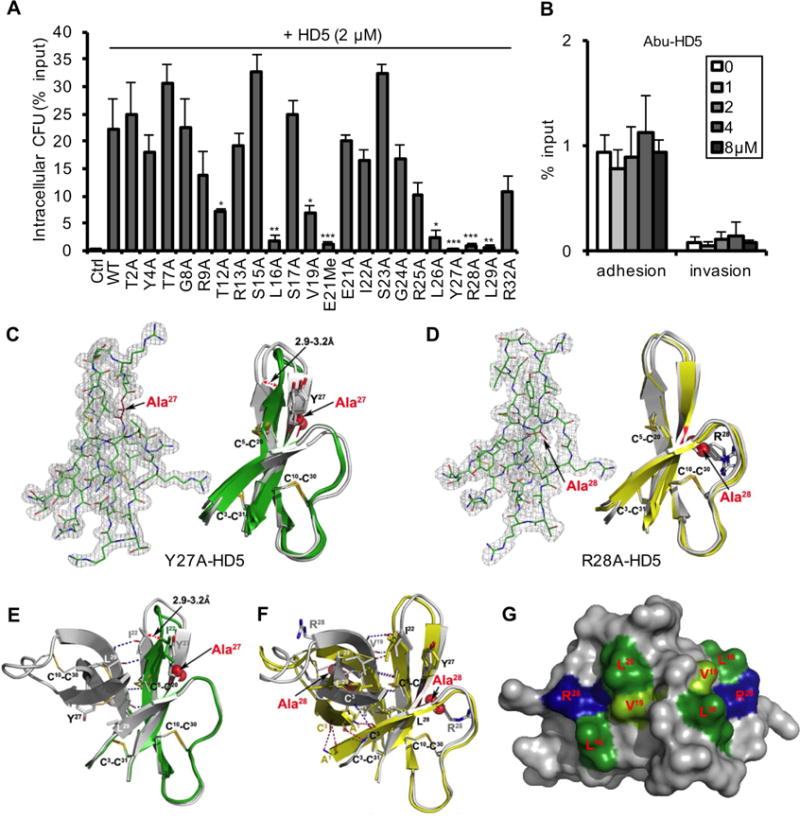
(A, B) Activities of native and alanine mutants of HD5 (A) and Abu-HD5 (B) on Shigella adhesion and invasion (for one hour). The adhesion and invasion assays were as in Fig. 1A, and data are expressed as the number of intracellular (A, B) and adhering (B) bacteria in HeLa cells relative to the input. Data are shown as mean ± SD of at least three independent experiments. Statistical significance in comparison with wildtype HD5 in A and in comparison with solvent control group in B was determined using a one-way ANOVA (Dunnett’s multiple comparison Test), and p values are as follows: *p < 0.05, **p < 0.01, ***p < 0.001. (C, D) The 2Fo-Fc electron density map contoured at 1.0σ of molecule A of Y27A-HD5 (C) and R28A-HD5 crystal (D) and the superimposition of defensin molecules present in the asymmetric units of analogs’ crystals with the wildtype HD5 monomers (shown in grey, from PDB code: 1ZMP (Szyk et al., 2006)). Side chains of cysteines forming disulfide bridges and mutated residues are shown as sticks. Structural analysis of Y27A-HD5 and R28A-HD5 analogs confirms that both mutant monomers assume the same fold as the wildtype HD5 monomer with no major changes to the overall structure and the network of three disulfide bridges. When superimposed, the root-mean-square deviations (RMSD) between 128 equivalent main chain atoms of wildtype HD5 and Y27A-HD5 and R28A-HD5 are in the range of 0.91–1.33 Å and 0.35–0.95 Å, respectively. (E, F) Crystal structures of the Y27A-HD5 monomer (E, green) and the R28A-HD5 dimer (F, yellow) superimposed on the wildtype HD5 dimer in grey (PDB code: 1ZMP). Mutated residues and alanine substitutions are shown as spheres. (G) Key functional residues of HD5 forming putative binding surfaces for interactions with bacterial and host proteins. Positively charged Arg28 residues are colored in blue, and hydrophobic residues Leu16, Val19, and Leu26 in green. Shades of green depict differences in activity with residues in light green being less important than those in dark green. Important residues not depicted in this view are Tyr27 and Leu29.
