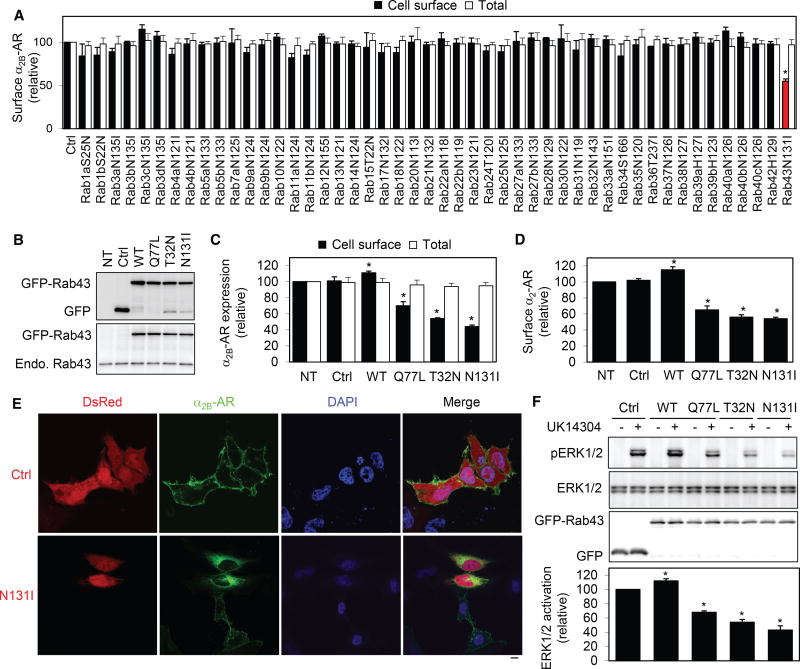
Figure 1. Effect of Rab43 and Its Mutants on the Cell-Surface Expression and Signaling of α2B-AR.
(A) Screening for Rab GTPases involved in the surface transport of α2B-AR. HEK293 cells stably expressing HA-α2B-AR were transfected with individual GFP-Rab mutants. The surface expression of α2B-AR was determined by intact cell ligand binding using [3H]-RX821002. The mean value of specific radioligand binding was 25,652 ± 1,745 cpm from cells transfected with the control vector.
(B) Western blot analysis of expression of Rab43 and its mutants by using GFP (top) and Rab43 antibodies (bottom). NT, non-transfection.
(C) Effect of Rab43 and its mutants on the surface expression of stably expressed HA-α2B-AR in HEK293 cells.
(D) Effect of Rab43 on the surface expression of endogenous α2-AR in MCF-7 cells. The mean value of specific ligand binding was 563 ± 67 cpm in MCF-7 cells without transfection.
(E) Effect of Rab43N131I on the subcellular distribution of α2B-AR. HEK293 cells were transfected with α2B-AR-GFP together with the vector dsRed-C1 (Ctrl) or dsRed-Rab43N131I. The subcellular distribution of α2B-AR was revealed by confocal microscopy.
(F) Effect of Rab43 on α2B-AR-mediated ERK1/2 activation. HEK293 cells stably expressing α2B-AR were transfected with the pEGFP-C1 vector (Ctrl) or GFPRab43. The cells were stimulated with UK14304 at 1 µM for 5 min, and ERK1/2 activation was determined by immunoblotting.
Data represent mean ± SE (n = 3–4) (A, C, D, and F). The images shown in (E) are representatives of at least 5 experiments. Scale bar, 10 µm. *p < 0.05 versus Ctrl.
See also Figures S1 and S2.