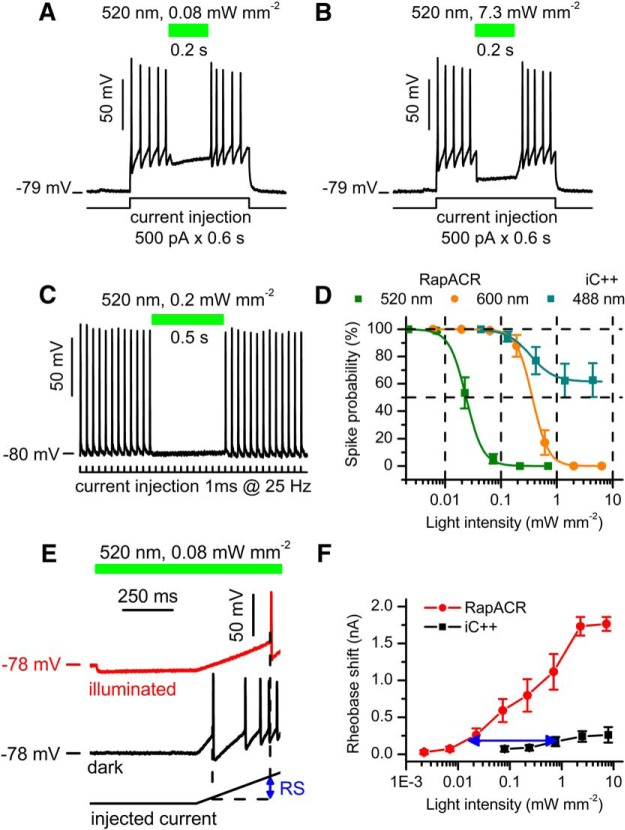
Figure 3.
RapACR is more efficient for neuronal silencing than the second-generation engineered Cl–-conducting channelrhodopsin iC++. A, B, Photoinhibition of spiking in a neuron expressing RapACR at two different light intensities. Spiking was induced by depolarization of the membrane by prolonged current injection as shown at the bottom. The time course of illumination is shown as green bars on top. C, A representative voltage trace recorded from a neuron expressing RapACR and stimulated with a train of 1-ms pulses of 2.5 nA delivered at 25 Hz. Passive response of the membrane (recorded under complete inhibition of spiking with light) was digitally subtracted. The time course of illumination is shown as a green bar. D, The dependence of neuronal inhibition on the light intensity for RapACR and iC++ photoactivated at different wavelengths. The data points are mean ± SEM (n = 15 and n = 14 neurons for RapACR and iC++, respectively) approximated with a logistic function; fitting parameters are listed in Table 4. E, Voltage traces recorded from a neuron transduced with RapACR and stimulated with a depolarizing current ramp (0–2 nA, 1 s; bottom trace) injected 500 ms after the onset of illumination (red) or in the dark (black). The blue arrow shows the photoinduced rheobase shift (RS). F, The dependence of the rheobase shift on the light intensity in neurons transduced with RapACR or iC++. The current ramp was from 0 to 2 nA in 1 s. The blue arrow shows the difference in the light sensitivity between the two tested channels. The data points are mean ± SEM (n = 9 and n = 6 neurons for RapACR and iC++, respectively).