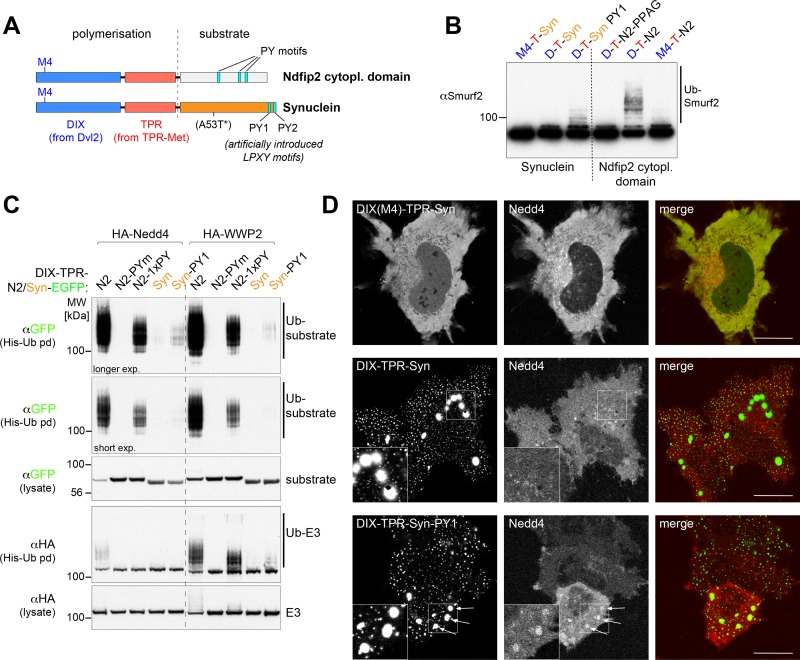
Fig 4. Clustering of alpha-synuclein does not result in efficient ubiquitination.
(A) Schematic of the polymerization domains (DIX and TPR) fused to a soluble substrate, either the cytoplasmic domain of Ndfip2 containing three PY motifs or full-length alpha-synuclein with mutants that were used in this study. The location of the A53T mutation is indicated, but mutant protein was not used in Fig 4 (see Fig 5A). (B) In vitro ubiquitination assays using Smurf2 with purified recombinant proteins as indicated above the panels, showing efficient Smurf2 autoubiquitination when incubated with polymerization-competent DIX-TPR-Ndfip2 with intact PY motifs (D-T-N2), but not when all three PY motifs were mutated (D-T-PPAG). No Smurf2 autoubiquitination was observed with DIX-TPR-alpha-synuclein (D-T-Syn) and only modest effects were observed with an introduced weak PY motif (D-T-Syn PY1). (C) Western blots of His pull downs from HEK293T cells co-transfected with His-Ub, HA-tagged Nedd4, WWP2 and the GFP-tagged highly efficient polymerizing DIX-TPR fusions as indicated. N2-Pym, Ndfip2 with all three PY motifs mutated; N2-1xPY, Ndfip2 with one remaining LPxY motif. (D) Live-cell images showing single confocal sections of representative HeLa cells transfected with DIX-TPR-alpha-synuclein-EGFP either with polymerization defective M4 mutant (top left), wild-type alpha-synuclein (middle left) or introduced weak PY motif (bottom left panel) and co-transfected with mCherry-Nedd4 (center panels). Recruitment of Nedd4 into larger punctate aggregates was observed only in the presence of the weak PY motif (see arrows). Insets show enlarged versions of the outlined areas.