Abstract
Background
No randomized trial has been conducted to directly compare enoxaparin with unfractionated heparin (UFH) in patients undergoing percutaneous coronary intervention (PCI) for non-ST-segment elevation acute coronary syndrome (NSTE-ACS). In an era where early invasive strategies are recommended in high risk patients, the effect of enoxaparin and UFH needs to be re-evaluated. The authors performed a meta-analysis to determine whether enoxaparin is superior to UFH in patients with NSTE-ACS undergoing PCI.
Methods
The composite efficacy end point included all-cause mortality and myocardial infarction (MI) in the hospital or within 60 days. Major bleeding, as defined in the individual clinical trials evaluated, was the main safety endpoint within the same time period. Pooled estimates of the difference in outcome between enoxaparin and UFH were calculated using fixed or random effects models.
Results
A total of 8,861 patients from 4 trials were included. In the pooled analysis, rates of death or MI were similar in patients treated with enoxaparin and UFH [risk ratio (RR), 0.89, 95% confidence interval (CI): 0.77–1.02, P=0.09; I2 =50%]. Major bleeding was also similar between enoxaparin and UFH (RR, 1.21, 95% CI: 0.94–1.56, P=0.15, I2=39%). A subgroup analysis, including randomized trials only or trials with a large sample size, and a leave-one-out sensitivity analysis, demonstrated similar results with above, respectively.
Conclusions
In patients undergoing PCI for NSTE-ACS, rates for both death/MI and major bleeding were similar between patients treated with enoxaparin and UFH.
Keywords: Enoxaparin, unfractionated heparin (UFH), acute coronary syndrome (ACS), percutaneous coronary intervention (PCI)
Introduction
Anticoagulation therapy, used concomitantly with antiplatelet therapy, has been proven to improve the prognosis of patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) (1). Comprehensive studies of patients with NSTE-ACS have shown that enoxaparin, a low-molecular-weight heparin (LMWH), is associated with a lower risk of adverse events compared with unfractionated heparin (UFH) (2-4).
However, no randomized trial has been conducted to directly compare enoxaparin and UFH in patients undergoing percutaneous coronary intervention (PCI) for NSTE-ACS. In an era where early invasive strategies are recommended in high risk patients, the effect of enoxaparin and UFH in the PCI setting needs to be re-evaluated. This analysis aims to determine whether enoxaparin is superior to UFH in patients with NSTE-ACS undergoing percutaneous revascularization.
Methods
Literature search strategy
We performed a literature search in the PubMed, MEDLINE, Web of Science, EMBASE, ClinicalTrials.gov, and the Cochrane Central Register of Controlled Trials databases from their inception until July 31, 2017. The following search formula was used: (unstable angina OR non-ST-segment elevation myocardial infarction OR non-ST-segment elevation acute coronary syndrome) AND (low molecular weight heparin OR unfractionated heparin OR enoxaparin) AND (angioplasty OR percutaneous coronary intervention). We also searched published abstracts presented at the meetings of the American Heart Association, the American College of Cardiology, and the European Society of Cardiology from their inception to 2017, as well as reference lists in relevant publications and abstracts. Language was restricted to English.
Study selection
Two independent reviewers (P He and Y Liu) scanned the titles and abstracts of all identified articles. Studies that were clearly unrelated were excluded at this stage. The same two reviewers independently assessed article eligibility. A third reviewer (L Jiang) resolved any disagreements between the two reviewers. Randomized trials and registry trials comparing enoxaparin with UFH in patients undergoing PCI for NSTE-ACS were included. A subgroup analysis of randomized trials was also included provided that data for the efficacy and safety end points were available. Moreover, studies were excluded if they (I) compared enoxaparin and UFH with placebo rather than compared the two agents head-to-head, or (II) enrolled patients with stable angina pectoris or ST-segment elevation myocardial infarction (STEMI) without reporting any specific data on NSTE-ACS.
Data extraction and assessment of risk of bias
Data extraction was performed by two independent reviewers. One reviewer (P He) extracted relevant data from the included studies, which were then checked by a second reviewer (Y Liu). The extracted data included the number of patients, population characteristics, enoxaparin dose and treatment duration, UFH dose and treatment duration, efficacy and safety end points, follow-up duration, and specific definition of bleeding.
Two reviewers (P He and Y Liu) independently evaluated the quality of the included studies. Disagreements were resolved by discussion and adjudicated by a third reviewer (L Jiang). We employed the Jadad scoring system to assess study quality (5) and the parameters applied included: (I) concealment of treatment allocation, (II) similarity of study groups at baseline, (III) eligibility criteria, (IV) use of any blinding procedure, (V) reporting of losses to follow-up, and (VI) intention to treat analysis (6).
End point definitions
The composite efficacy end point included all-cause death and myocardial infarction (MI) in the hospital or within 60 days. MI incidence was not available within this time period from the Korea Acute Myocardial Infarction Registry (KAMIR) trial even after we contacted the corresponding author; therefore, only mortality in this trial was calculated. Major bleeding, as defined by the individual studies, was the main safety endpoint within the same time period.
Statistical analysis
Data were analyzed using Review Manager version 5.2. (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) and STATA version 11 (StataCorp, College Station, TX, USA). Comparison of the treatment effect between enoxaparin and UFH on clinical outcomes was reported as a risk ratio with 95% confidence intervals (95% CI). The Q statistic was calculated and heterogeneity was quantified using the I2 statistic. We regarded I2 ≤25%, 25–50%, and >50% as low, moderate, and high heterogeneity, respectively. Random-effect models were used when I2 >50%; otherwise, a fixed-effect model was employed. A funnel plot was used to assess publication bias, while a subgroup analysis was conducted based on the study design (randomized or registry) and sample size. A small sample size was defined as trials enrolling <500 patients and a large sample size, as those enrolling >500 patients. Finally, trial sequential analysis (TSA) was performed to decrease the risk of type I errors, and determine whether the present evidence is reliable and conclusive (7,8). For this TSA, we estimated the required information size using α =0.05 (two sided) and β =0.20 (power 80%). The event proportions in UFH were pooled by using a random effect meta-analysis model with logic transformation of proportions, and an absolute risk reduction of 1–3% for death or MI and increase of 1–1.5% for major bleeding in the enoxaparin group, considering the clinical significance of the outcomes. TSA was performed using TSA software 0.9.5.5 Beta (Copenhagen Trial Unit, Copenhagen, Denmark) (9). All tests were two-tailed and P<0.05 was considered statistically significant in the meta-analysis.
Results
A total of 1,532 articles were screened according to the search strategy. After removing duplicates, 1,397 studies remained, of which 1,144 were discarded after the review of the title and abstract. Consequently, 253 full-text articles were assessed for eligibility. After applying the exclusion criteria, four articles with a total of 8,861 patients were included in the final analysis (Figure 1) (10-13). Studies such as ACUTE II (3) were excluded due to a lack of data pertaining to patients undergoing PCI.
Figure 1.
Study flow chart.
Moreover, two articles were subgroup analyses of randomized controlled trials (n=5,131) and the other two were observational studies (n=3,730). Specific data on patients undergoing PCI in the ESSENCE trial and the TIMI 11B trial were pooled by Fox et al. in one article; thus, four studies were included in this meta-analysis. The flow chart of the search strategy is shown in Figure 1.
Baseline characteristics of the included studies and interventions
A total of 4,041 (45.6%) and 4,820 (54.4%) patients were treated with enoxaparin and UFH, respectively. Major baseline characteristics are shown in Tables 1,2. Previous history of MI was more frequent in patients who received enoxaparin in the SYNERGY study (enoxaparin 27.6% vs. UFH 25.0%, P=0.045), whereas in the KAMIR study, previous MI was more frequent in patients treated with UFH (enoxaparin 7.0% vs. UFH 10.0%, P=0.008) (Tables 1,2). In the studies of ACS (13), patients in the enoxaparin group were more frequently female, were older, and had a higher incidence of comorbidities, including hypertension, diabetes mellitus, and previous stroke. Moreover, they were more likely to receive clopidogrel within 48 hours after admission (Tables 1,2). The manner of administration of the two anticoagulants is shown in Tables 1,2. The time period for follow up varied, with one study collecting pertinent events at 30 days, another at 43 days, and the other two before discharge. Additional data from the included studies is shown in Table S1.
Table 1. Baseline characteristic of patients and interventions in included studies.
| Author, years | No. of patients, enoxaparin/UFH | Inclusion, criteria | Enoxaparin/UFH |
|---|---|---|---|
| Keith, 2002 | 201/244 | Unstable angina or non-ST-segment elevation MI | ESSENCE: enoxaparin 1 mg/kg, twice daily. UFH intravenous bolus of 5,000 IU followed by continuous dose-adjusted infusion |
| TIMI 11B: enoxaparin initial intravenous bolus of 30 mg followed by twice daily subcutaneous injections of 1 mg/kg. UFH initial bolus of 70 IU/kg followed by continuous infusion of 15 IU/kg/h. All patients were given intravenous UFH to achieve an ACT of ≥350 seconds | |||
| Harvey, 2006 | 2,323/2,364 | High-risk patients with acute coronary syndrome | Enoxaparin (1 mg/kg) was given subcutaneously 12 hourly. Before PCI, no supplemental enoxaparin was recommended if the last dose was administered <8 hours previously and 0.3 mg/kg of supplemental enoxaparin was given if the last enoxaparin dose had been given ≥8 hours. UFH was administered intravenously (60 IU/kg bolus and initial infusion of 12 IU/kg/h) with a target activated partial thromboplastin time of 50 to 70 seconds or 1.5 to 2.0 times the upper limit of normal. At the time of PCI, Additional intravenous UFH was given to achieve an ACT of 250 seconds |
| Zeymer, 2006 | 339/994 | Subgroup of high-risk patients with acute coronary syndrome without ST elevation | No details |
| Li, 2012 | 1,178/1,219 | Non-ST-segment elevation myocardial infarction | Enoxaparin: subcutaneous injection of 1 mg/kg b.i.d. During PCI, a reduced dose of UFH (50 U/kg) was given to those who received enoxaparin within 8 h before PCI to maintain activated clotting time 200 |
| UFH: 24,000 U/day infusion after their arrival at the hospitals. During PCI, 70–100 U/kg was given to maintain the target ACT of 250–300 s |
UFH, unfractionated heparin; MI, myocardial infarction; PCI, percutaneous coronary intervention; ACT, activated clotting time.
Table 2. Baseline characteristic of patients in included studies.
| Author, years | Age, (years), enoxaparin/UFH | Females (%), enoxaparin/UFH | Diabetes (%), enoxaparin/UFH | Hypertension (%), enoxaparin/UFH | HF (%), enoxaparin/UFH | Dyslipidemia (%), enoxaparin/UFH | Previous MI (%), enoxaparin/UFH |
|---|---|---|---|---|---|---|---|
| Keith, 2002 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Harvey, 2006 | 67.0/67.0 | 32.0/30.7 | 26.1/26.6 | 66.3/64.8 | 6.6/6.9 | 58.2/60.1 | 27.6/25.0 |
| Zeymer, 2006 | 72.4/71.9 | 41.8/37.7 | 36.2/31.7 | 78.3/75.6 | NA | 68.9/67.368.9/67.3 | 30.3/28.9 |
| Li, 2012 | 63.8/63.7 | 32.6/30.5 | 33.2/30.8 | 55.3/52.7 | 3.1/2.5 | 12.6/13.5 | 7.0/10.0 |
UFH, unfractionated heparin; MI, myocardial infarction; HF, heart failure.
Table S1. Additional data of included studies.
| Study, year | Randomization time | Blinding | Key exclusion criteria | Definitions | Event adjudication | |
|---|---|---|---|---|---|---|
| Major bleeding | Myocardial infarction | |||||
| Keith, 2002 | Acute phase | NA | A | TIMI criteria | B | NA |
| Harvey, 2006 | NA | NA | NA | TIMI criteria | C | D |
| Zeymer, 2006 | Non RCT | NA | NA | NA | NA | NA |
| Li, 2012 | Non RCT | NA | E | F | G | NA |
A, Exclusion criteria included the presence of a left bundle-branch block or pacemaker, persistent ST-segment elevation, angina with an established precipitating cause (e.g., heart failure or tachydysrhythmia), contraindications to anticoagulation, or a creatinine clearance rate of less than 30 mL per minute; B, defined by electrocardiogram and serum cardiac markers criteria; C, the diagnosis of periprocedural MI required a total CK or CK-MB level >3 times the upper limit of normal and at least 50% above the preprocedural level; D, a clinical events committee blinded to the patients' randomization; E, STEMI, NSTEMI with bare metal stenting or without stenting, contraindication to antithrombotic agents, known bleeding disorders, thrombocytopenia (<100×109/L), administration of oral anticoagulants, conservative treatment without PCI, infarction related to the grafted vessel, and estimated life expectancy of less than 12 months; F, major bleeding was defined as any intracranial bleeding, bleeding associated with the need for blood transfusion, or any other clinically relevant bleeding as judged by the investigator; G, recurrent myocardial infarction was defined as the development of either pathologic Q waves in at least two contiguous leads or an increase in the creatine kinase level to more than twice the upper limit of normal with an elevation of creatine kinase-MB isoenzyme.
Assessment of study quality and publication bias
The quality of the included randomized controlled trials was moderate. None of the studies provided concealment of allocation. Three studies enrolled patients with similar baseline characteristics and provided details for the eligibility criteria and completeness of follow-up. None of the studies described the randomization methods or provided any details for evaluation of the appropriateness of randomization. Two studies (ESSENCE and TIMI 11B) by Fox et al. reported blinding of both patients and researchers to treatment assignment. The intention-to-treat analysis was observed in all four studies.
The funnel plot was relatively symmetrical (Figure S1), and the result of Egger’s test confirmed the absence of obvious publication bias among the included trials for primary efficacy and safety outcomes (all P>0.05).
Figure S1.
Funnel plot for the subjective assessment of bias among the included studies. RR, risk ratio; MI, myocardial infarction.
Composite end point (death or MI)
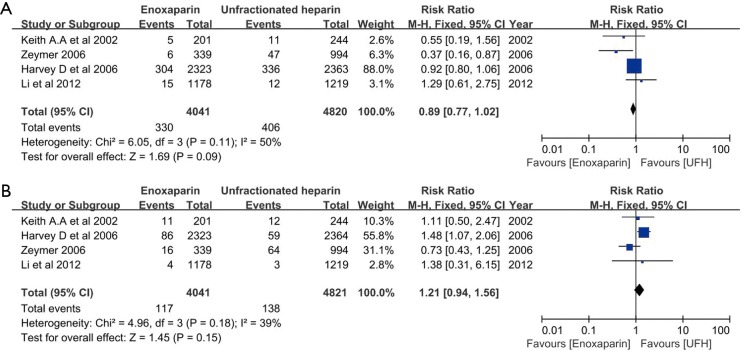
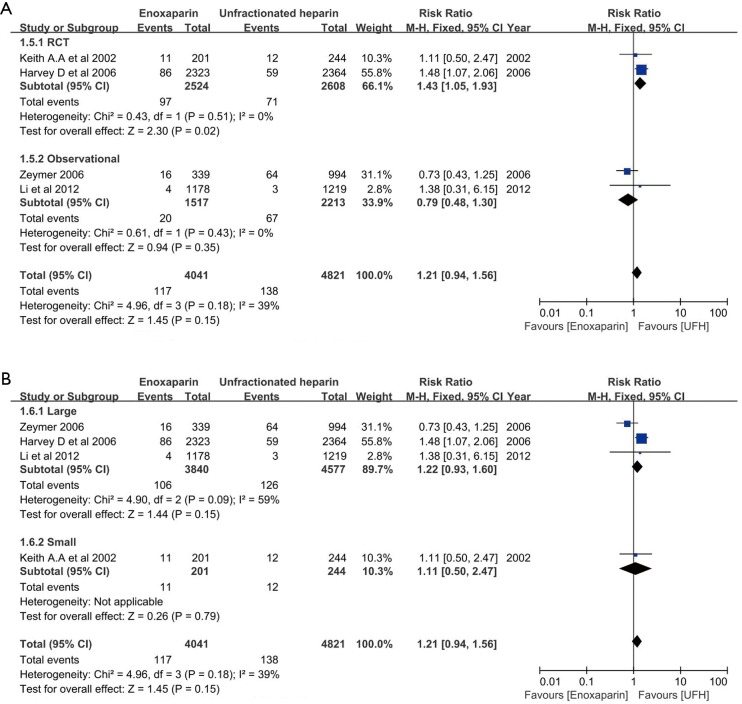
In the overall cohort of patients (n=8,861), the composite end point was comparable between patients treated with enoxaparin and UFH (0.89, 95% CI: 0.77–1.02, respectively; P=0.09; I2 =50%) (Figure 2). Even after excluding the non-randomized trial, no difference in the incidence of death/MI between patients treated with enoxaparin and those treated with UFH was noted (0.91, 95% CI: 0.79–1.05, respectively; P=0.19; I2=0) (Figure 3A). Analysis of trials enrolling >500 patients showed a similar result (0.90, 95% CI: 0.78–1.03, respectively; P=0.13; I2 =61%) (Figure 3B).
Figure 2.
Enoxaparin vs. unfractionated heparin for the comparison of death or myocardial infarction (A) and major bleeding (B).
Figure 3.
Subgroup analysis for the comparison of death or myocardial infarction based on study design (A) and sample size (B).
Major bleeding
No significant difference in the incidence of major bleeding was observed between patients treated with enoxaparin and UFH (1.21, 95% CI: 0.94–1.56, respectively; P=0.15, I2=39%) (Figure 2). However, after excluding the non-randomized trial, the difference in major bleeding became statistically significant enoxaparin and UFH treatment (1.43, 95% CI: 1.05–1.93, respectively; P=0.02; I2 =0%) (Figure 4A). The risk of major bleeding in patients who received enoxaparin also tended to be higher than in those who received UFH in the analysis including trials with a large sample size (1.22, 95% CI: 0.93–1.60, respectively; P=0.15; I2 =59%) (Figure 4B).
Figure 4.
Subgroup analysis for the comparison of major bleeding based on study design (A) and sample size (B).
Sensitivity analysis
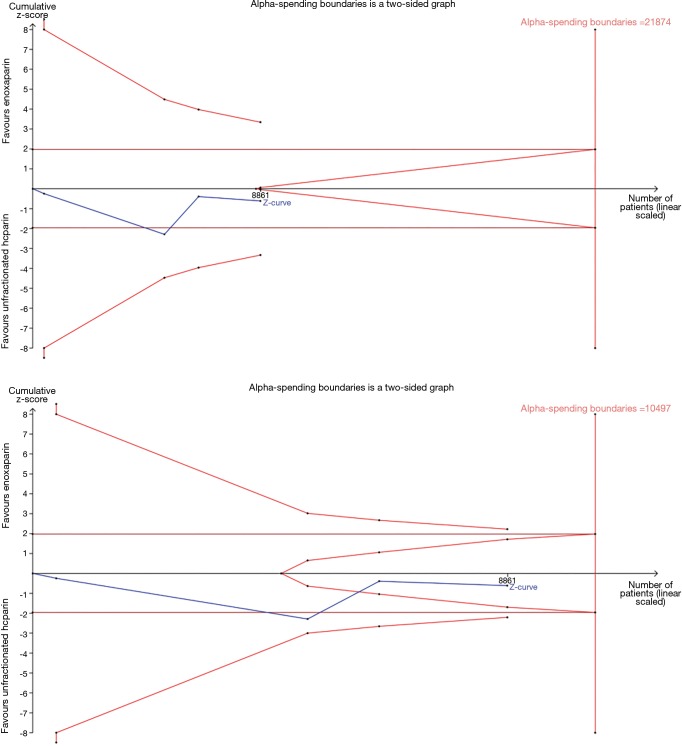
TSA showed that the risk of a type 2 error was minimal, and the meta-analysis was conclusive for a 3% reduction in death/MI, and a 1.5% increase in major bleeding. However, a larger sample size is needed for conclusive results regarding reductions of 1% or 2% in death/MI, and an increase of 1% for major bleeding (Figures S2,S3).
Figure S2.
Results of trial sequential analysis for death or myocardial infarction (for reduction of 1%, 2%, 3%, respectively).
Figure S3.
Results of trial sequential analysis for major bleeding (for reduction of 1%, and 1.5%, respectively).
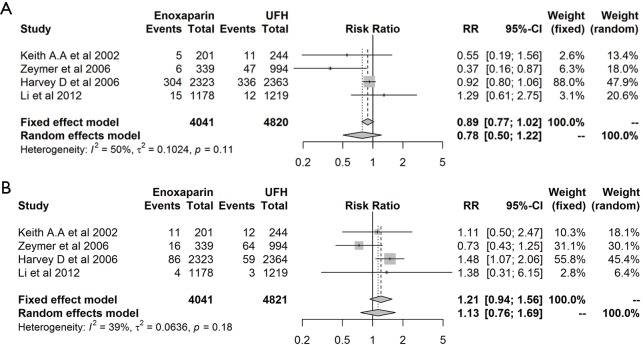
The leave-one-out sensitivity analysis revealed that our results were sufficiently robust. The RR and 95% CI regarding the effect of enoxaparin and UFH on death/MI (Figure 5A) and major bleeding (Figure 5B) were similar to previous results.
Figure 5.
Leave-one-out sensitivity analysis.
Discussion
To the best of our knowledge, our study is the first meta-analysis to compare the effect of enoxaparin and UFH in patients undergoing PCI for NSTE-ACS. This meta-analysis demonstrated that both death/MI and major bleeding were similar between the patient groups.
Anticoagulation therapy, together with antiplatelet therapy, plays a crucial role in the treatment of NSTE-ACS. UFH had been the predominant anticoagulant in this setting until new anticoagulation agents emerged (14-16). Among the alternatives to UFH, LMWH, especially enoxaparin, has been comprehensively studied and proven to be more effective in reducing coronary ischemic events. This is mainly attributed to its more predictable anticoagulant effect, lower risk of immune-mediated thrombocytopenia, and greater specific inhibition of Factor Xa (17,18). Moreover, ESSENCE and TIMI 11B were two major trials that established the superiority of enoxaparin over UFH in NSTE-ACS patients who were primarily treated with a conservative approach. Evidence from these trials contributed to the recommendation of anticoagulation therapy with enoxaparin in the American College of Cardiology/American Heart Association (Class I-A) and the European Society of Cardiology (Class I-B) guidelines (19,20). However, it should be noted that these trials were completed almost 20 years ago, when PCI was not considered as standard practice despite the majority of patients enrolled being considered high-risk.
Early invasive strategy has been increasingly applied in NSTE-ACS patients presenting with high-risk characteristics (21,22). Considering this trend in clinical practice, the benefit of enoxaparin in NSTE-ACS patients for early invasive management should be evaluated. Contrary to the results of a previous meta-analysis for the general NSTE-ACS population, our analysis found that enoxaparin was not associated with a lower mortality/MI in an invasively managed population compared with UFH (23). This result was supported by the subgroup analysis in the A-Z trial (2), which was, however, excluded from our meta-analysis because of a lack of data for the individual end points needed for analysis. In the A-Z trial, although a subset analysis of patients with a planned conservative strategy for treatment showed that enoxaparin is associated with a lower incidence of the composite primary end point (death, MI, and refractory ischemia) compared with UFH, the advantage of enoxaparin was not apparent in patients randomized to early invasive strategy. Moreover, it should be noted that in both the TIMI 11B and ESSENCE trials, early PCI was discouraged and the improvement of outcomes in the enoxaparin arm was mainly driven by the reduction of refractory ischemia and MI. Hence, the benefit of anticoagulation in reducing recurrent angina and MI in patients undergoing coronary revascularization being mitigated by an invasive procedure to immediately restore blood flow may not be surprising. This could also explain the findings of the other two studies (SYNERGY and KAMIR) included in our analysis, which only focused on patients receiving invasive management at the index admission (10,11).
In addition to the increasing role of invasive management, decreasing the time from admission to PCI might also contribute to the attenuation of the advantage of enoxaparin use. In the SYNERGY trial, which found no superiority of enoxaparin over UFH, the median time from randomization to PCI was approximately 22 hours and was markedly less than that of the TIMI 11B and ESSENCE trials. Timely revascularization could certainly reduce the risk of recurrent angina and the need for urgent revascularization therapy, thereby attenuating the beneficial effect of aggressive anticoagulation. Moreover, data from the TIMI 11B and ESSENCE trials suggested that enoxaparin possibly has a time-dependent beneficial effect. In these two trials, no advantage was found in any individual efficacy end point in the enoxaparin group within the first 48 hours after administration. In patients undergoing PCI, discontinuation of anticoagulation therapy after revascularization could result in insufficient therapy time, which may consequently prevent enoxaparin from achieving an advantage over UFH.
Apart from the trend to early invasive management, progress in antiplatelet therapy could also influence the efficacy of anticoagulation in NSTE-ACS. Platelet activation and adhesion have been recognized as an essential step for atherothrombosis, and dual antiplatelet therapy (DAPT) has been recommended in an attempt to inhibit platelet aggregation. No patients in the TIMI 11B and ESSENCE trials received DAPT; however, approximately 30% of patients in the SYNERGY trial and >99% in the KAMIR studies did. It could be expected that the benefit of aggressive anticoagulation would decrease when DAPT is routinely used in patients with NSTE-ACS.
While the incidence of death/MI was similar between the enoxaparin and UFH groups, this meta-analysis found that enoxaparin tends to be associated with a higher risk of major bleeding compared with UFH. However, this should be interpreted with caution because of crossover anticoagulation and the lack of a unified definition of major bleeding across the studies. The SYNERGY trial was the only trial that showed a difference in major bleeding between enoxaparin and UFH (enoxaparin 3.7% vs. UFH 2.5%, P=0.028) (10). It is noteworthy that in this trial, up to 14.6% of patients in the enoxaparin group received additional UFH; however, the difference in major bleeding did not reach statistical significance when patients who had crossover anticoagulation therapy were excluded (enoxaparin 3.1% vs. UFH 2.4%, P=0.154). Post-randomization crossover anticoagulation therapy without monitoring might expose the patient to excessive anticoagulation, which in turn would make the precise determination of the respective effect of the two anticoagulants on bleeding challenging. In the KAMIR study, no excessive bleeding was found in patients receiving enoxaparin as the initial anticoagulation therapy despite the routine administration of additional UFH during PCI. This might be explained by the routine monitoring of activated clotting time (ACT) to guide anticoagulation therapy not only in the UFH arm but also in the enoxaparin arm. Cavusoglu et al. demonstrated that ACT could be used to evaluate the anticoagulation level of enoxaparin (24); hence, ACT monitoring during PCI might be helpful to avoid over-anticoagulation and to reduce the risk of bleeding when additional UFH is routinely used. The minimum target ACT during PCI in the KAMIR study was set at 200 seconds, which coincided perfectly with that suggested by Marmur et al. (25). Moreover, trials included in this meta-analysis each had their own specific definition of major bleeding, which possibly resulted in some bias when combining the data.
Furthermore, another possible underlying reason for the similar bleeding incidence for enoxaparin and UFH in the KAMIR study was the higher proportion of transradial PCI. While <10% of cases in the SYNERGY trial used a radial approach, the number of cases increased to approximately 40% in Korea in the KAMIR study (26). Transradial PCI could dramatically reduce access site bleeding, thereby making the previously described difference in bleeding events between enoxaparin and UFH not significant. In the KAMIR study, although >99% of patients received DAPT, the major bleeding incidence was notably lower (0.3% for enoxaparin and 0.2% for UFH) than that of the SYNERGY trial (3.7% for enoxaparin and 2.5% for UFH), in which clopidogrel was only administered to approximately 30% of the study population.
Limitations
This meta-analysis has several limitations that need to be addressed. First, the small sample size was a major limitation that could lead to bias. However, we collected all of the data available according to the inclusion criteria, focusing on patients undergoing PCI for NSTE-ACS. TSA demonstrated that a well-designed, large, randomized controlled trial was needed to provide sufficient and convincing evidence on the role of enoxaparin and UFH. Secondly, the various definitions of major bleeding used in the studies complicated the interpretation of the results pertaining to that safety end point. Third, the different durations of anticoagulation treatment in the trials added to the complexity of precisely comparing the two anticoagulants.
Conclusions
This meta-analysis suggests that in NSTE-ACS patients undergoing PCI, both mortality/MI and major bleeding are similar between patients treated with enoxaparin and patients treated with UFH.
Acknowledgements
The authors appreciate the efforts of their statistical consultant, Chong-yang Duan of the Department of Biostatistics, Southern Medical University, China.
Funding: This study was supported by Science and Technology Planning Project of Guangzhou City (201707010002), Guangzhou, China, and Medical Science and Technology Research Funding of Guangdong (A2017347), and Cardiovascular Health Funding of Beijing (LHJJ201611016).
Ethical Statement: All analyses were based on previously published studies, thus no ethical approval or patient consent were required.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Eikelboom JW, Anand SS, Malmberg K, et al. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet 2000;355:1936-42. 10.1016/S0140-6736(00)02324-2 [DOI] [PubMed] [Google Scholar]
- 2.Blazing MA, de Lemos JA, White HD, et al. Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA 2004;292:55-64. 10.1001/jama.292.1.55 [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Theroux P, Borzak S, et al. Randomized double-blind safety study of enoxaparin versus unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes treated with tirofiban and aspirin: the ACUTE II study. The Antithrombotic Combination Using Tirofiban and Enoxaparin. Am Heart J 2002;144:470-7. 10.1067/mhj.2002.126115 [DOI] [PubMed] [Google Scholar]
- 4.Goodman SG, Fitchett D, Armstrong PW, et al. Randomized evaluation of the safety and efficacy of enoxaparin versus unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes receiving the glycoprotein IIb/IIIa inhibitor eptifibatide. Circulation 2003;107:238-44. 10.1161/01.CIR.0000050144.67910.13 [DOI] [PubMed] [Google Scholar]
- 5.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 6.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41. 10.1016/S0895-4356(98)00131-0 [DOI] [PubMed] [Google Scholar]
- 7.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64-75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Whitehead A, Simmonds M. Sequential methods for random- effects meta-analysis. Stat Med 2011;30:903-21. 10.1002/sim.4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorlund K, Engstrøm J, Wetterslev J, et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark. 2011. p.1-115. Available online: www.ctu.dk/tsa [Google Scholar]
- 10.White HD, Kleiman NS, Mahaffey KW, et al. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial. Am Heart J 2006;152:1042-50. 10.1016/j.ahj.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 11.Li YJ, Rha SW, Chen KY, et al. Low molecular weight heparin versus unfractionated heparin in patients with acute non-ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention with drug-eluting stents. J Cardiol 2012;59:22-9. 10.1016/j.jjcc.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 12.Fox KA, Antman EM, Cohen M, et al. Comparison of enoxaparin versus unfractionated heparin in patients with unstable angina pectoris/non-ST- segment elevation acute myocardial infarction having subsequent percutaneous coronary intervention. Am J Cardiol 2002;90:477-82. 10.1016/S0002-9149(02)02517-1 [DOI] [PubMed] [Google Scholar]
- 13.Zeymer U, Gitt A, Junger C, et al. Clinical benefit of enoxaparin in patients with high-risk acute coronary syndromes without ST elevations in clinical practice. Am J Cardiol 2006;98:19-22. 10.1016/j.amjcard.2006.01.047 [DOI] [PubMed] [Google Scholar]
- 14.Théroux P, Waters D, Qiu S, et al. Aspirin versus heparin to prevent myocardial infarction during the acute phase of unstable angina. Circulation 1993;88:2045-8. 10.1161/01.CIR.88.5.2045 [DOI] [PubMed] [Google Scholar]
- 15.Neri Serneri GG, Modesti PA, Gensini GF, et al. Randomised comparison of subcutaneous heparin, intravenous heparin, and aspirin in unstable angina. Studio Epoorine Sottocutanea nell'Angina Instobile (SESAIR) Refrattorie Group. Lancet 1995;345:1201-4. 10.1016/S0140-6736(95)91990-2 [DOI] [PubMed] [Google Scholar]
- 16.Oler A, Whooley MA, Oler J, et al. Adding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina. A meta-analysis. JAMA 1996;276:811-5. 10.1001/jama.1996.03540100055028 [DOI] [PubMed] [Google Scholar]
- 17.Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular- weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001;119:64S-94S. 10.1378/chest.119.1_suppl.64S [DOI] [PubMed] [Google Scholar]
- 18.Silvain J, Beygui F, Ankri A, et al. Enoxaparin anticoagulation monitoring in the catheterization laboratory using a new bedside test. J Am Coll Cardiol 2010;55:617-25. 10.1016/j.jacc.2009.08.077 [DOI] [PubMed] [Google Scholar]
- 19.Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. 10.1093/eurheartj/ehr236 [DOI] [PubMed] [Google Scholar]
- 20.Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST- elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2012;126:875-910. 10.1161/CIR.0b013e318256f1e0 [DOI] [PubMed] [Google Scholar]
- 21.Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009;360:2165-75. 10.1056/NEJMoa0807986 [DOI] [PubMed] [Google Scholar]
- 22.Montalescot G, Cayla G, Collet JP, et al. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 2009;302:947-54. 10.1001/jama.2009.1267 [DOI] [PubMed] [Google Scholar]
- 23.Murphy SA, Gibson CM, Morrow DA, et al. Efficacy and safety of the low-molecular weight heparin enoxaparin compared with unfractionated heparin across the acute coronary syndrome spectrum: a meta-analysis. Eur Heart J 2007;28:2077-86. 10.1093/eurheartj/ehm224 [DOI] [PubMed] [Google Scholar]
- 24.Cavusoglu E, Lakhani M, Marmur JD. The activated clotting time (ACT) can be used to monitor enoxaparin and dalteparin after intravenous administration. J Invasive Cardiol 2005;17:416-21. [PubMed] [Google Scholar]
- 25.Marmur JD, Bullock-Palmer RP, Poludasu S, et al. Avoiding intelligence failures in the cardiac catheterization laboratory: Strategies for the safe and rational use of dalteparin or enoxaparin during percutaneous coronary intervention. J Invasive Cardiol 2009;21:653-64. [PubMed] [Google Scholar]
- 26.Park KH, Jeong MH, Ahn Y, et al. The impact of vascular access for in-hospital major bleeding in patients with acute coronary syndrome at moderate- to very high-bleeding risk. J Korean Med Sci 2013;28:1307-15. 10.3346/jkms.2013.28.9.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]