Abstract
Background
The aim of the study is to analyse 5-year survival and prognostic factors in patients operated due to lung cancer with postoperatively confirmed metastases to N2 group nodes.
Methods
In the years 2007–2015, 1,148 patients with lung cancer were treated surgically. A postoperative histopathological study confirmed N2 feature in 150 patients. One hundred and ten patients, in whom a 5-year survival analysis was possible to perform, were included in such analysis. The patients underwent the following procedures: pneumonectomy, 31 patients; lobectomy, 61; bilobectomy, 10; and wedge resection, 8. All patients were subjected to supplementary treatment after the surgery.
Results
Five-year survival was achieved in 23 patients (21%). The patients’ survival did not depend on the type of surgery, type of tumour, its location or presence of metastases in N1 nodes (P=0.82, P=0.51, P=0.36 and P=0.23, respectively). A statistically significant correlation was observed (P=0.01) between the 5-year survival of a patient and the occurrence of metastases only in one group of lymph nodes of the N2 feature (22 patients, 20%). Involvement of three or more mediastinal nodal groups resulted in survival shorter than 5-year.
Conclusions
(I) In patients with the N2 feature, the type of performed surgery, type of tumour and the occurrence of metastases in the lymph nodes of the lung hilum do not affect 5-year survival; (II) involvement of only one nodal group allows to achieve 5-year survival in 20% of patients; (III) involvement of three and more nodal groups with the N2 feature results in decreased 5-year survival.
Keywords: Non-small cell lung cancer (NSCLC), N2 disease, surgery, survival, prognostic factors
Introduction
In recent years, malignant tumours have become a serious worldwide epidemiological threat. Lung cancer is the main oncological cause of death in men, and the third one in women. This disease mostly affects inhabitants of North America, eastern Asia, north-east Europe, Australia and New Zealand. In 2012, this pathology contributed to over 1.6 million deaths worldwide. It is estimated that in 2035, this number will reach 3 million (2.1 million men and 0.9 million women) (1). On setting a diagnosis, about 30% of patients have already demonstrated local progression of the disease and future treatment is determined by the type and number of lymph nodes involved by the tumour (2). Patients with III A stage make up a heterogeneous group. Thus, the size of the tumour, invasion of adjacent structures and invasion of lymph nodes of the N1 or N2 feature are factors which allow to classify the patients in this particular group (3). Ruckdeschel divided the patients with the N2 feature into three groups. The first one includes patients, in whom the invasion of mediastinal lymph nodes is invisible and detected only in postoperative material. The second group includes patients with confirmed metastases in the N2 group nodes, detected by imaging or surgical procedures (mediastinoscopy, EBUS, EUS). The third group includes patients with multi-level, large N2 nodes (4). The influence of the N2 feature is indisputable and determines the percentage of 5-year survivals. The aim of the study is an analysis of factors that affect the survival in patients with non-small cell lung cancer (NSCLC) with the N2 feature, confirmed with postoperative examination.
Methods
Study population
In the years of 2007–2015, 1,148 patients with lung cancer were included in treatment. A postoperative histopathological study confirmed the N2 feature in 150 patients. Before the surgery, all patients had undergone chest CT and bronchoscopy. In the case of lymphadenopathy (the diameter of lymph node in short axis exceeds 10 mm), PET-CT, mediastinoscopy was performed as well as EBUS from 2008. The patients who did not demonstrate metastases to lymph nodes or those with only one type of lymph nodes, affected by the tumour and in whom the nodes were not clumped into packages, were qualified for the procedure.
Definition of group
Out of the total number of 150, we excluded 37 patients whose postoperative lifespan was shorter than 5 years. We also rejected 3 patients, who died in the perioperative period due to cardiological reasons. Finally, 110 patients were included into the analysis. The material was collected from 61 men and 49 women, aged from 40 to 78 years (the mean age was 62.2 years). Imaging studies showed presence of tumour in the right lung in 46 patients, and in the left lung in 64 patients. Histopathologically, the tumours were classified as adenocarcinoma in 57 cases, squamous cell carcinoma in 42 cases and large cell carcinoma in 11 cases.
Surgical procedure
Anatomic resections (lobectomy, bilobectomy pneumonectomy) of lung tissue with en bloc removal of lymph nodes at stations 2R, 4R, 7, 8, 9 and 10R for right-sided tumours and nodes at stations 4L, 5, 6, 7, 8, 9 and 10L for left-sided tumours were performed. We decided to remove at least 6 groups of nodes with N1 and N2 feature in all patients. A wedge resection was considered only in patients with low spirometry values. The stage of the tumour was evaluated according to the TNM classification (UICC 2009-7 edition), while the collected lymph nodes were classified according to the Naruke map (5). All patients underwent supplementary treatment after the surgery.
Statistical analysis
The statistical analysis included the following factors: the size and histological type of the tumour, its location, extent of the performed surgery, number of metastatic lymph nodes and presence of skip metastases. We did not take into consideration postoperative chemo- and radiotherapy. It will be a subject of another study. The overall survival (OS) of the patients was defined as a period of time between the surgery and death due to various reasons. Kaplan-Meier probability curves of survival were calculated using the Gehan-Wilcoxon test. In order to analyse the age of patients, we used the t-test. The Mann-Whitney test was used to measure the size of the tumour, whereas, the chi square test was used to calculate values of other variables.
Results
Treatment courses of patients with N2 disease
Table 1 presents patients’ characteristics. The tumour was located in the right upper lobe in 13 patients, middle lobe in 2 patients, right lower lobe in 29 patients, left upper lobe in 28 patients and left lower lobe in 38 patients. Of the total number of 110 patients, 61 underwent lobectomy, 10 bilobectomy, 31 pneumonectomy and 8 wedge resection.
Table 1. Patients’ characteristics.
| Parameter | n [%] |
|---|---|
| Number of patients | 110 |
| Age (median/range) | 62/40–78 |
| Sex | |
| Male | 69 [63] |
| Female | 41 [37] |
| Tumor location | |
| Lobe left upper | 28 [25] |
| Left lower | 38 [35] |
| Right upper | 13 [12] |
| Middle | 2 [2] |
| Right lower | 29 [26] |
| Histopathology | |
| Adenocarcinoma | 57 [52] |
| Squamous cell carcinoma | 42 [38] |
| Multicellular carcinoma | 11 [10] |
| Type of procedure | |
| Lobectomy | 61 [56] |
| Bilobectomy | 10 [9] |
| Pneumonectomy | 31 [28] |
| Wedge resection | 8 [7] |
| Clinical T factor | |
| T1 | 29 [26] |
| T2 | 65 [59] |
| T3 | 14 [13] |
| T4 | 2 [2] |
| Pathological N status | |
| Skip metastases | 23 [21] |
| Single station N2 | 22 [20] |
| Multi-station N2 | 88 [80] |
Lobectomy/bilobectomy was the most frequent procedure (71 patients), performed mainly at stages T1a–T2b (48/71). Pneumonectomies were most often performed for tumours located in the left lung (25/31), mainly at stages T2a–b and T3. Wedge resections were performed at stages T1a–T1b in patients with low spirometry values.
Final pathological T and N status
An analysis of postoperative material showed advancement of T2 in 65 patients (59%). Twenty-nine patients were operated on in stage T1, 14 in T3 and only 2 in T4.
Jumping metastases were reported in 23 patients (21%). The N2 feature was found only within one node group, in 22 patients. In one case, the advancement of N2 concerned two groups. In the remaining 87 cases, metastases were found in three or more nodal groups. In 44 patients with neoplasm located in the right lung, metastases were most often found in subcarinal lymph nodes (node No. 7 according to Naruke) and in lower paratracheal lymph nodes (node No. 4 according to Naruke): 28 cases. With regards to left lung tumours, metastases were most commonly found in subcarinal and subaortic lymph nodes (nodes No. 7 and 5 according to Naruke: 37 cases) and in para-aortic lymph nodes (node No. 6 according to Naruke: 20 cases). A detailed location of N2 metastases is shown in Table 2.
Table 2. Metastases in groups of mediastinal lymph nodes according to Naruke map.
| N2 feature | n | Average survival (months), N2/N0 | P |
|---|---|---|---|
| Right lung (n=44) | |||
| Group No. 2 | 4 | x | x |
| Group No. 3 | 1 | x | x |
| Group No. 4 | 28 | 17/48 | 0.0148 |
| Group No. 7 | 47 | 29/36 | 0.4480 |
| Group No. 8 | 5 | x | x |
| Group No. 9 | 6 | x | x |
| Left lung (n=66) | |||
| Group No. 4 | 15 | 17/ 36 | 0.2946 |
| Group No. 5 | 37 | 24/ 36 | 0.7268 |
| Group No. 6 | 20 | 13/34 | 0.3269 |
| Group No. 7 | 37 | 12/57 | 0.0008 |
| Group No. 8 | 1 | x | x |
| Group No. 9 | 9 | x | x |
x, small numbers—making a statistical analysis is not possible.
Results in patients with the N2 feature after surgery
Five-year survival was achieved by 23 patients (21%). The patients’ survival was not related to the type of surgery, type of tumour, its location or occurrence of metastases in N1 nodes (P=0.55, P=0.55 and P=0.23, respectively) Table 3. The OS of patients is presented in Figure 1. A statistically significant correlation was observed (P=0.01) between 5-year survival of a patient and the presence of metastases only in one group of lymph nodes of the N2 feature (22 patients, 20%). Involvement of three or more mediastinal nodal groups resulted in survival shorter than 5-year (on average 12.5 months) (Table 3). The analysis also showed a reduced time of survival, on average to 17 months, in patients with right lung tumours, accompanied by metastases to group No. 4 of nodes (right paratracheal according to Naruke), P=0.01 (Figure 2). With regards to patients with left lung tumours, accompanied by metastases to subcarinal lymph nodes—group No. 7 (P=0.0008), the survival decreased, on average, to 12 months (Table 2 and Figure 3). In patients with jumping metastases, the 5-year survival rate was observed in 35% of cases but the value was statistically insignificant (P=0.25).
Table 3. Location of tumour, histopathology and type of procedure versus 5-year survival.
| Parameter | % of 5-year survival (%) | P |
|---|---|---|
| Tumour location | 0.3655 | |
| Lobe upper left | 9/28 (32.14) | |
| Lower left | 7/38 (18.42) | |
| Upper right and middle | 3/15 (20.00) | |
| Lower right | 4/29 (13.79) | |
| Histopathology | 0.5173 | |
| Adenocarcinoma | 11/57 (19.30) | |
| Squamous cell carcinoma | 11/42 (26.19) | |
| Large cell carcinoma | 1/11 (9.09) | |
| Type of procedure | 0.8271 | |
| Lobectomy | 13/61 (21.31) | |
| Bilobectomy | 3/10 (30.00) | |
| Pneumonectomy | 6/31 (19.35) | |
| Wedge resection | 1/8 (12.50) | |
| T feature | 0.0824 | |
| T1 | 10/29 (34.48) | |
| T2 | 12/65 (18.46) | |
| T3 | 1/14 (7.14) | |
| T4 | 0/2 (0) | |
| N1 feature/skip metastases | 0.25 | |
| Skip metastases | 8/23 (34.78) | |
| N1 | 15/71 (21.13) | |
| N2 feature | 0.01 | |
| 1 group N2 | 22 (20.0) | |
| 2 group N2 | 1 (0.9) | |
| 3 group N2 and more | 0 (0.0) |
Figure 1.
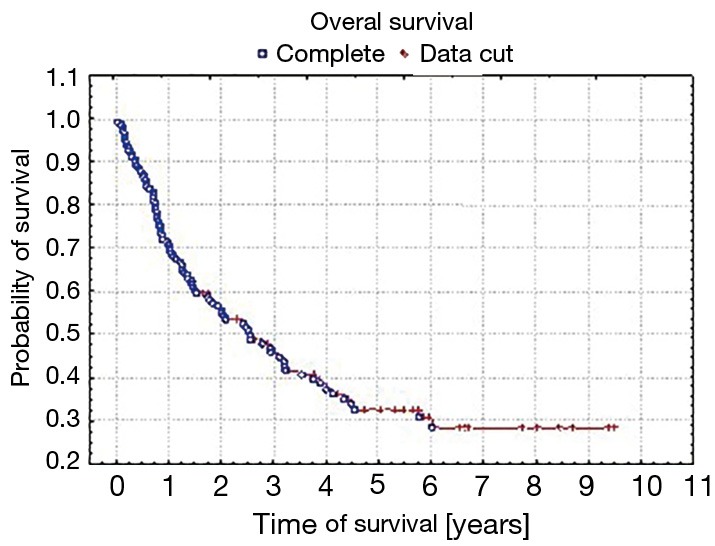
The overall survival of patients with N2 disease.
Figure 2.
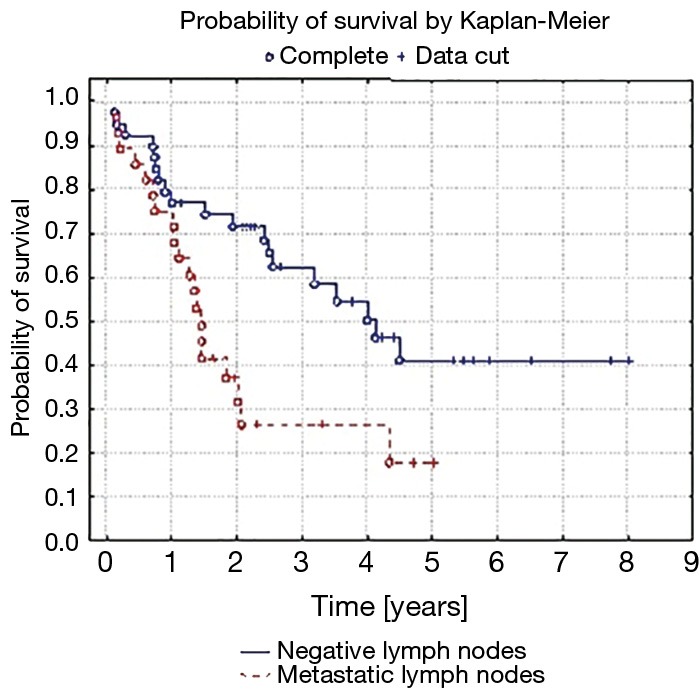
Probability of survival by Kaplan-Meier of patients with metastases in right paratracheal lymph nodes (group No. 4 according to the Naruke map). P=0.01.
Figure 3.
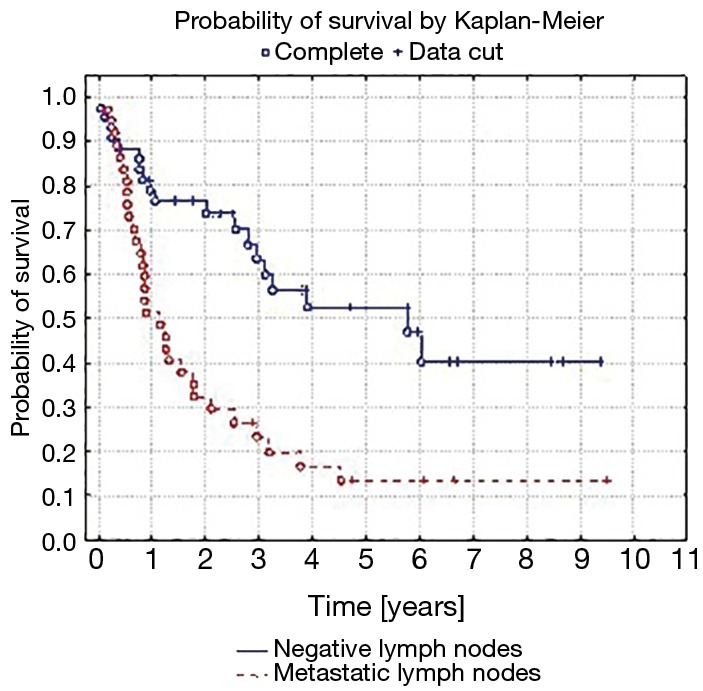
Probability of survival by Kaplan-Meier of patients with metastases in subcarinal lymph nodes (group No. 7 according to the Naruke map). P=0.0008.
Discussion
Indications for surgical treatment of patients with stage III A/N2 are not really clear. The decision on implementation of treatment depends on the heterogeneity of patients with the N2 feature and subsequent prognosis (6). In most cases, patients at this stage are not treated surgically but they are administered chemo- and/or radiotherapy. Surgical treatment is administered only to a selected group of patients, and involves implementing chemo-radiotherapy or chemo-radiotherapy with surgery treatment system (7). A randomised test of Intergroup trial 0139 indicates that such method of disease management is justified. A study by Albain et al. showed a prolonged disease free survival (DFS) in patients who received radio-chemotherapy after a surgical treatment. There were no differences in OS when exclusively chemotherapy or radio-chemotherapy were applied (8). In a study published in 2016 by Lei et al., another correlation was observed. The two- and three-model treatment system did not show any differences in DFS, while OS was significantly prolonged in patient subject to postoperative radio-chemotherapy (P=0.006) (9).
Factors determining DFS and OS which are most often cited in literature include: the size and histological type of tumour, its location, type of treatment, number of metastatic lymph nodes (4,7,10,11,12). There is a close, unquestionable relationship between tumoral size and risk of metastases to lymph nodes. In the event of tumours ranging from 3cm to 5cm in diameter, the N0 feature is expected to occur in 48% of patients. In the case of tumours larger than 7 cm, the above feature will be probably found in only 1/3 of patients (13). Bao et al. showed that the N2 feature can be positive (13%) in patients with the T1a feature (11). In our study, in patients at stages T1, T2 and T3, the percentage of 5-year survival was 34%, 18% and 7%, respectively; however, the difference in the groups was not statistically significant (P=0.08).
The diagnosis of adenocarcinoma significantly increased the risk of involved mediastinal lymph nodes (11,12). Xiong et al. conducted a multivariable analysis, which showed that the histopathological type of tumour, in addition to the patient’s sex and size of the tumour, is a significant factor determining the N2 feature (14). Ilic et al. found an increased percentage of N2 nodes pathologically affected in adenocarcinoma (15). In our study, patients with squamous cell cancer lived longer than patients with adenocarcinoma (26% versus 19%).
Some authors claim that the location of the tumour is a potential risk factor of metastases to lymph nodes of the hilum and mediastinum (11,14,16). Bao et al. examined patients with the T1a feature. They showed a statistically significant relationship (P=0006) between the positive N2 feature and the location of the tumour in the lower or middle lobe (11). In turn, Qiang et al. noted a higher percentage of non-regional N2 in tumours located also in the lower lobe (16). In a study published in 2016, Xiong et al. showed a statistically significant correlation (P<0.01) between the occurrence of metastases to lymph nodes of the aortic and lower mediastinum and a tumour with the feature from T1a to T2b, located in the upper left lobe. A similar significant relationship was observed in the case of metastases to the upper mediastinum, when tumours with the above feature were located in the upper right lobe (14). In our study, patients with cancer located in upper lobes lived longer than those with tumours located in lower lobes. However, the groups were not statistically different (P=0.36).
In the case of advanced N2, lobectomy is the most preferred type of treatment. Kawasaki et al. showed that 5-year survival at this stage of clinical advancement was 31.5% for lobectomy, 25% for bilobectomy, and only 15.6% for pneumonectomy (7). Also, Albain et al. observed an adverse effect of pneumonectomy on OS (8). In the presented analysis, the highest percentage of 5-year survivals was achieved after bilobectomy (30%). For lobectomy, the percentage was 21%, whereas for pneumonectomy, it was 19%.
The most important determinants of distant survival in lung cancer patients are metastases to lymph nodes of the N2 group. The authors point out that the N2 feature itself is not homogenous, and the prognosis depends on the number of involved lymph nodes, their type and the occurrence of skip metastases (15-18). Patients with the N2 feature, manifesting with involvement of only one lymph group, are best candidates for a surgery.
The authors point out that the clinical stage of patients with one group of N2 nodes (single-station N2) does not have to correspond to real advancement (true single-station N2) (17,18). Five-year patient survival in such cases is estimated to be 23 (28%) (18,19). In our material, single metastases to the N2 group were observed in 22 patients, in whom 5-year survival was 20%.
Some authors emphasise the importance of subcarinal lymph nodes as they might be prognostic indicators of distant survivals. Hishida et al. observed 5-year survival in 44% of patients with the N2 feature, when it included only one nodal group and this was not the subcarinal node (16,18). Our analysis confirmed that occupation of subcarinal nodes, only in tumours located in the left lung, was an unfavourable prognostic factor (P=0.0008). With regards to right lung tumours, involvement of paratracheal lymph nodes (No. 4 according to Naruke) contributed to poor prognosis (P=0.0148). The occurrence of multiple N2 metastases (multiple N2 station) significantly (P=0.001) deteriorates the prognosis, and 5-year survival decreases to 11% (16,19). Studies have shown that occupation of more than three N2 node groups is a significant (P<0.001) factor deteriorating the prognosis, while the 5-year survival is around 5% (16). In the analysed group of patients, we obtained similar results. Metastases in over three N2 groups were a factor, which did not allow to achieve 5-year survival (P=0.01). Bearing in mind the number and type of metastatic N2 lymph nodes, some authors decided to propose a revision of the TNM classification in the aspect of the N2 features (20). The advocates of this innovation believe that such an improvement will allow to more accurately qualify patients for surgeries and predict prognosis in patients operated on for grade III lung cancer (20,21).
Many authors also point out a significant impact of skip metastases on the length of survival (15,16,22). Such metastases are frequently observed in squamous cell carcinoma (15). However, in the study performed on patients with adenocarcinoma, Li et al. showed skip metastases in 25% of patients. The 5-year survival rate of these patients was 60.7%. Ilic et al. observed skip metastases in 46% of patients with squamous cell carcinoma. Nevertheless, the postoperative survival of these patients did not differ from the survival of those, in whom the N2 feature was observed (15). In the group of patients included in the analysis, the occurrence of skip metastases resulted in 5-year survivals observed in 35% of patients; with regards to the statistical significance, P=0.25.
Conclusions
In patients with the N2 feature, the type of performed operation, the type of cancer and occurrence of metastases in lymph nodes of the lung hilum do not affect 5-year survival;
Occupation of only one nodal group allows to obtain 5-year survival in 20% of patients;
Occurrence of metastases in paratracheal lower right and subcarinal lymph nodes significantly reduces the survival time.
Acknowledgements
None.
Ethical Statement: The study was approved by Ethics Committee of the Medical University of Lodz (No. RNN/233/18/KE) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Didkowska J, Wojciechowska U, Mańczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150. 10.21037/atm.2016.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marulli G, Verderi E, Zuin A, et al. Outcomes and prognostic factors of non-small-cell lung cancer with lymph node involvement treated with induction treatment and surgical resection. Interact Cardiovasc Thorac Surg 2014;19:256-62. 10.1093/icvts/ivu141 [DOI] [PubMed] [Google Scholar]
- 3.Terán MD, Brock MV, Staging lymph node metastases from lung cancer in the mediastinum. J Thorac Dis 2014;6:230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruckdeschel JC, Combined modality therapy of non-small cell lung cancer. Semin Oncol 1997;24:429-39. [PubMed] [Google Scholar]
- 5.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. 10.1378/chest.111.6.1718 [DOI] [PubMed] [Google Scholar]
- 6.Horinouchi H, Goto Y, Kanda S, et al. Candidates for Intensive Local Treatment in cIIIA-N2 Non-Small Cell Lung Cancer: Deciphering the Heterogeneity. Int J Radiat Oncol Biol Phys 2016;94:155-62. 10.1016/j.ijrobp.2015.09.026 [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki K, Sato Y, Suzuki Y, et al. Prognostic factors for surgically resected N2 non-small cell lung cancer. Ann Thorac Cardiovasc Surg 2015;21:217-22. 10.5761/atcs.oa.14-00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III for non-small-cell lung cancer: a phase III randomized controlled trial. Lancet 2009;374:379-86. 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei T, Xu X-L, Chen W, et al. Adjuvant chemotherapy plus radiotherapy is superior to chemotherapy following surgical treatment of stage IIIA N2 non-small-cell lung cancer. Onco Targets Ther 2016;9:921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uehara H, Nakao M, Mun M, et al. Significant prognostic factors for completely resected pN2 Non-small cell lung cancer without neoadjuvant therapy. Ann Thorac Cardiovasc Surg 2015;21:345-53. 10.5761/atcs.oa.14-00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao F, Yuan P, Yuan X, et al. Predictive risk factors for lymph node metastasis in patients with small size non-small cell lung cancer. J Thorac Dis 2014;6:1697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong J, Wang R, Sun Y, et al. Clinical analysis of sixty-four patients with T1aN2M0 stage non-small cell lung cancer who had undergone resection. Thoracic Cancer 2016;7:215-21. 10.1111/1759-7714.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Chen H, Xiang J, et al. Relationship between tumour size and disease stage in non-small cell lung cancer. BMC Cancer 2010;10:474-9. 10.1186/1471-2407-10-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong J, Wang R, Sun Y, et al. Lymph node metastasis according to primary tumour location T1 and T2 stage non-small cell lung cancer patients. Thoracic Cancer 2016;7:304-9. 10.1111/1759-7714.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilic N, Petricevic A, Arar D, et al. Skip mediastinal nodal metastases in the IIIa/N2 non-small cell lung cancer. J Thorac Oncol 2007;2:1018-21. 10.1097/JTO.0b013e318158d471 [DOI] [PubMed] [Google Scholar]
- 16.Qiang G, Liang C, Yu Q, et al. Risk factors of recurrence after complete resection of pathological stage N2 non-small cell lung cancer. Thoracic Cancer 2015;6:166-71. 10.1111/1759-7714.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller SM, Vangel MG, Wagner H, et al. Prolonged survival in patients with resected non-small lung cancer and single-level N2 disease. J Thorac Cardiovasc Surg 2004;128:130-7. 10.1016/j.jtcvs.2003.11.061 [DOI] [PubMed] [Google Scholar]
- 18.Hishida T, Yoshida J, Ohe Y, et al. Surgical outcomes after initial surgery for clinical-station N2 non-small cell lung cancer. Jpn J Clin Oncol 2014;44:85-92. 10.1093/jjco/hyt164 [DOI] [PubMed] [Google Scholar]
- 19.Inoue M, Sawabata N, Takeda S, et al. Results of surgical intervention for p-stage IIIA (N2) non-small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumour of upper lobe. J Thorac Cardiovasc Surg 2004;127:1100-6. 10.1016/j.jtcvs.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 20.Saji H, Tsuboi M, Shimada Y, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013;143:1618-25. 10.1378/chest.12-0750 [DOI] [PubMed] [Google Scholar]
- 21.Matsunaga T, Suzuki K, Takamochi K, et al. Time to refine N2 staging? cN2α and cN2β based on local regional involvement provide a more accurate prognosis in surgically treated IIIA non-small-cell lung cancer than N2 alone or the number of node stations involved. Eur J Cardiothorac Surg 2014;46:86-91. 10.1093/ejcts/ezt550 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Hu H, Wang R, et al. Lung adenocarcinoma: are skip N2 metastases different from non-skip? J Thorac Cardiovasc Surg 2015;150:790-5. 10.1016/j.jtcvs.2015.03.067 [DOI] [PubMed] [Google Scholar]


