Figure 2.
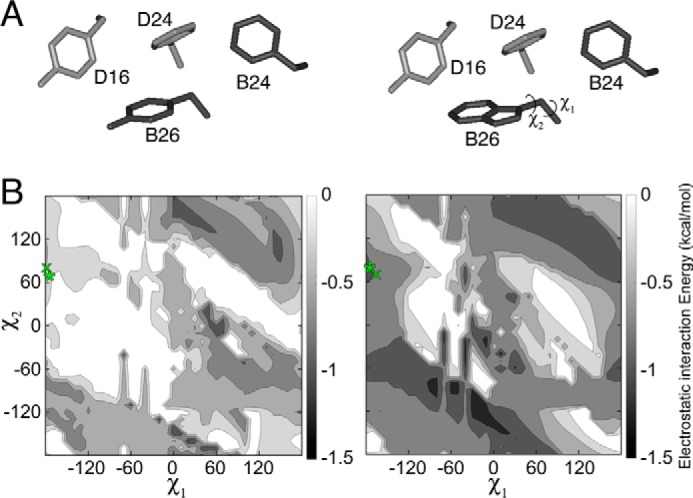
Molecular simulations of aromatic interactions in the insulin dimer. A, aromatic–aromatic interactions across insulin's dimer interface involve PheB24, TyrB16, PheD24 (sticks) and either TyrD26 (left) or TrpD26 (right). Residues were extracted from T6 structure 4INS. B, contour maps depicting empirical interaction energies between B26 (Tyr on left and Trp on right) at varying χ1 and χ2 angles and the other three residues shown in A. The orientation of TyrB26 or TrpB26 in the WT or variant crystal structure is indicated by a green “x”; orientation of TyrB26 or TrpB26 in the local model is indicated by a green asterisk.
