Figure 5.
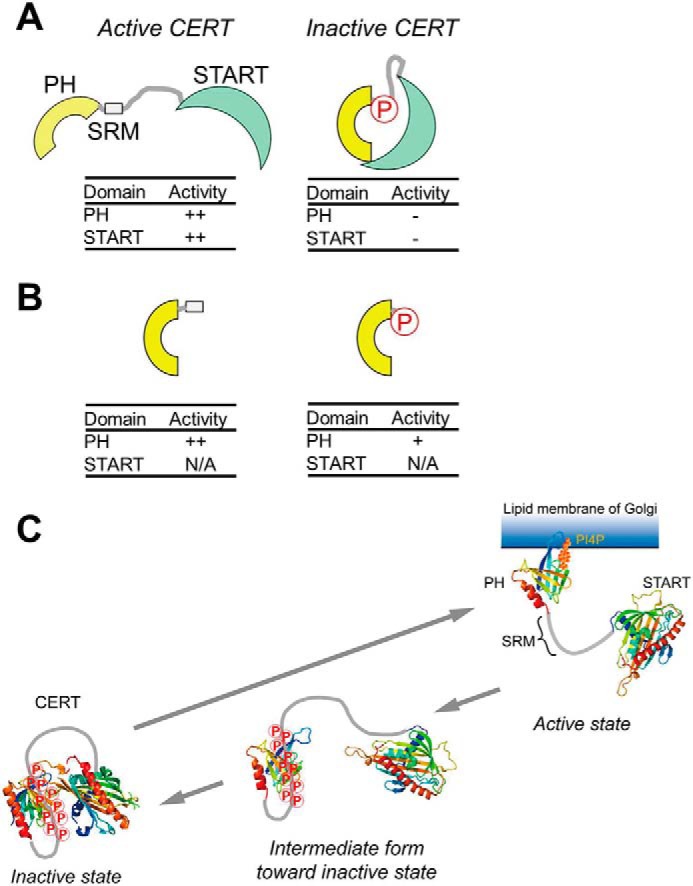
A schematic model of the phosphoregulation of the structure and function of CERT. The hyperphosphorylation of SRM induces a conformational change in CERT to mutually mask the N-terminal PH domain and C-terminal START domain (A), as described in a previous study (9). On the other hand, as new findings in the present study, we demonstrate that phosphorylated SRM may also partially attenuate the function of the PH domain by directly interacting with the PH domain (B). N/A, not applicable. C, a functional diagram of the structural and functional regulation of CERT based on previously reported insights (A) and the results of the present study (B). On the Golgi membrane, the SRM of CERT is hyperphosphorylated by the Golgi-resident protein kinase PKD or CKIγ (9). The interaction between hyperphosphorylated SRM and the CERT PH domain (PDB code 2RSG) may facilitate the dissociation of CERT from the PI4P-embedded Golgi membrane. After dissociation from the Golgi membrane, CERT with hyperphosphorylated SRM may undergo a more drastic conformational rearrangement toward a state in which PH-START interdomain mutual functional silencing may be attained (9). The interaction between hyperphosphorylated SRM and the CERT PH domain identified in this study would be a first step of the conformational rearrangement of the CERT to lead to functional regulation. Notably, whereas the interaction between the PH domain and phosphorylated SRM occurs in an intramolecular manner, the interaction between the PH and START domains (PDB code 5JJD) may occur between different monomers in a CERT homooligomer (also see “Discussion”). Nevertheless, the schematic model tentatively illustrates only the intramolecular interaction between the two domains in a CERT monomer for simplicity.
