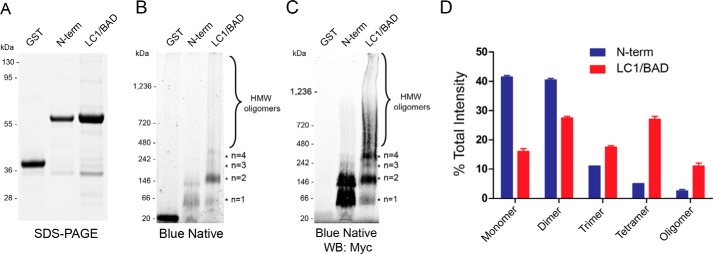
Figure 2.
LC1/BAD domain of U1-70K directly self-interacts and oligomerizes in vitro. A, SDS-PAGE of GST alone, purified N-terminal domain (N-term), and purified LC1/BAD domain of rU1-70K. Both the LC1/BAD and N-term domains have equivalent molecular masses (∼65 kDa), whereas GST alone is (∼20 kDa). B, BN-PAGE of GST alone, the N-terminal domain, and the LC1 domain of rU1-70K, respectively. The LC1/BAD domain formed higher molecular weight species (*) consistent with dimers (∼130 kDa), trimers (∼195 kDa), tetramers (∼260 kDa), and high-molecular weight (HMW) oligomers (>400 kDa). C, Western blotting detection of blue native complexes using Myc antibodies. D, densitometry of monomeric, dimeric, trimeric, and high-molecular weight species of the N-term domain (blue) and LC1/BAD domain (red) of rU1-70K. Each form is represented as the fraction of total signal intensity in each sample analyzed in technical replicate (n = 2). Error bars represent standard deviation.