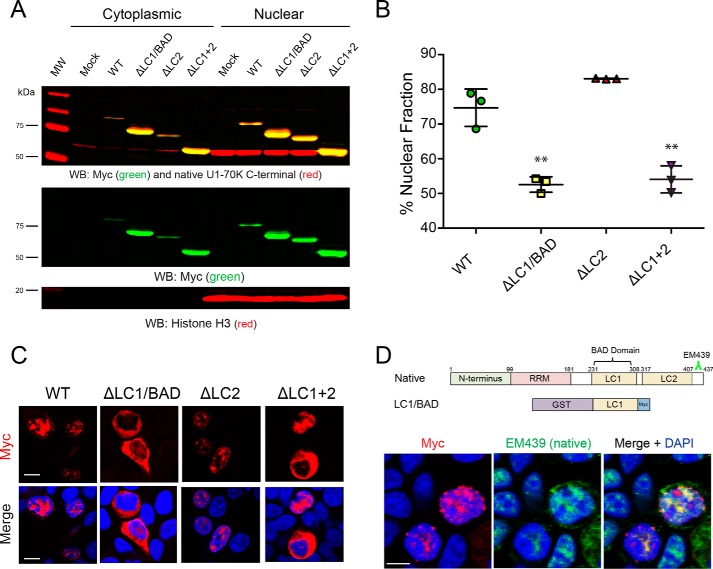
Figure 3.
LC1/BAD domain of U1-70K is necessary and sufficient for nuclear granule localization. A, WT full-length rU1-70K or variants lacking one or both LC domains were overexpressed in HEK293 cells. The cells were then fractionated into nuclear and cytoplasmic pools followed by Western blot (WB) analysis for both recombinant Myc-tagged proteins (green) and native U1-70K (red). Western blottings for histone H3 (bottom panel) were used as a positive control in the nuclear fraction. B, densitometry analysis was performed to calculate the levels of cytoplasmic and nuclear WT rU1-70K and variants, and the percent nuclear intensity for each rU1-70K protein is reported. Each experiment was performed in biological triplicate (n = 3) with error bars representing the standard deviation (S.D.). Both the ΔLC1 and ΔLC1 + 2 rU1-70K fragments were significantly less nuclear than full-length rU1-70K (**, p value <0.01 by ANOVA compared with WT). C, immunocytochemistry for WT rU1-70K and deletion variants that lacked the LC1/BAD, LC2, or both LC domains was performed and visualized by confocal microscopy. Scale bar equates to 10 μm. D, overexpression of the rU1-70K LC1/BAD domain alone (red) resulted in nuclear granule association and sequestration of native U1-70K (green). The EM439 antibody detects an extreme C-terminal epitope not present in the LC1/BAD rU1-70K protein, which allows discrimination between the recombination protein and native U1-70K. DAPI-stained nuclei are shown in blue. Scale bar equates to 10 μm.