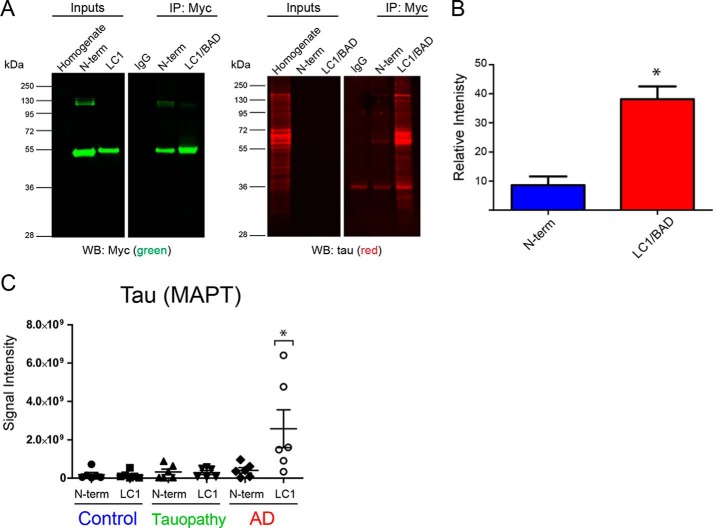
Figure 9.
LC1/BAD domain of U1-70K interacts with Tau specifically in AD brain. A, GST-purified N-terminal domain or the LC1/BAD domain (4 μg) of rU1-70K was added separately to AD brain homogenates and immunoprecipitated with anti-Myc antibodies. IP with a nonspecific IgG was also performed as a negative control. Inputs and IPs were analyzed by Western blotting using anti-Tau antibodies (red) and Myc antibodies (green). B, LC1/BAD domain interacted with significantly higher levels of Tau from AD brain than the N-terminal domain (t test one-tail p value = 0.0156). The experiment was done in biological triplicate (n = 3) from independent AD cases with the mean and standard deviation shown. C, GST-purified N-terminal fragment or the LC1 domain (4 μg) of rU1-70K was added separately to brain homogenates from control (n = 6), AD (n = 6), or non-AD tauopathy (n = 6) brain tissue and immunoprecipitated with anti-Myc antibodies followed by mass spectrometry (MS) analysis. Label-free quantification was used to determine the signal intensities (y axis) of Tau across co-IP conditions (LC1 or N-term) and brain tissue homogenates. The mean and standard deviations for each condition are shown. Statistical significance for Tau interactions with the LC1 and N-terminal domain was determined by ANOVA. Significantly more Tau interacted with the LC1 domain in AD brain homogenates compared with all other experimental conditions (*, p value <0.05).