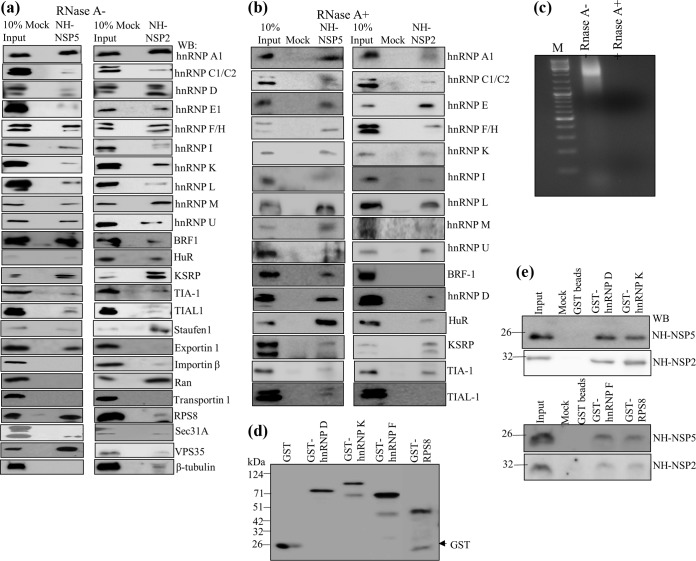
FIG 1.
Interaction of cellular hnRNPs and ARE-BPs in RNase-treated and untreated cell extracts with purified recombinant viral nonstructural proteins NSP2 and NSP5 demonstrated by a pulldown assay. The recombinant NH-tagged viral proteins NSP5 and NSP2 were purified from E. coli by affinity chromatography using Ni2+-NTA-agarose beads. Control Ni2+-NTA-agarose beads, which were prepared by passing the lysate from E. coli harboring the pET22-NH vector lacking the viral gene, were used for mock binding. Both the experimental and control beads were further incubated in binding buffer containing 0.5% BSA to minimize the nonspecific binding of cellular proteins. (a and b) The RNase-treated purified recombinant NSP2 and NSP5 proteins bound to Ni2+-NTA-agarose beads, and the control beads (mock binding) were incubated with equal amounts (500 μg) of control MA104 cell extracts that were either not treated with RNase (a), similar to what was done for mass spectrometry, or treated with RNase (b). The cellular proteins bound to the beads were resolved by SDS-PAGE, and the interacting cellular proteins were detected by immunoblotting. In the lane representing 10% input, 50 μg of the RNase-treated or untreated cell extracts was loaded. The same blot was used to detect two or three host proteins by sequential deprobing and reprobing depending on clear differences in the molecular weights of the proteins. Each PD assay was repeated at least 3 to 4 times to confirm reproducibility. (c) The cell extracts (1 mg/ml) were incubated with 100 μg of RNase A for 45 min at room temperature, and 100 μg of the RNase-treated and untreated cell extracts was resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. Note the complete digestion of cellular RNA in the RNase-treated extract. M, molecular marker. (d) Expression and purification of GST-tagged recombinant host proteins. The bacterial cell extracts were incubated with RNase A (100 mg/ml) prior to purification. (e) Demonstration of direct interactions of purified NH-NSP2 and NH-NSP5 with glutathione bead-bound GST-tagged nuclear proteins. Ten micrograms of purified NH-NSP2 or NH-NSP5 was incubated with approximately 5 μg of the bead-bound recombinant GST-tagged hnRNPDp40 isoform and hnRNP K (top) and hnRNP F and RPS8 (bottom) treated further with RNase A (10 mg/ml), and the bound viral protein was detected by Western blotting (WB).