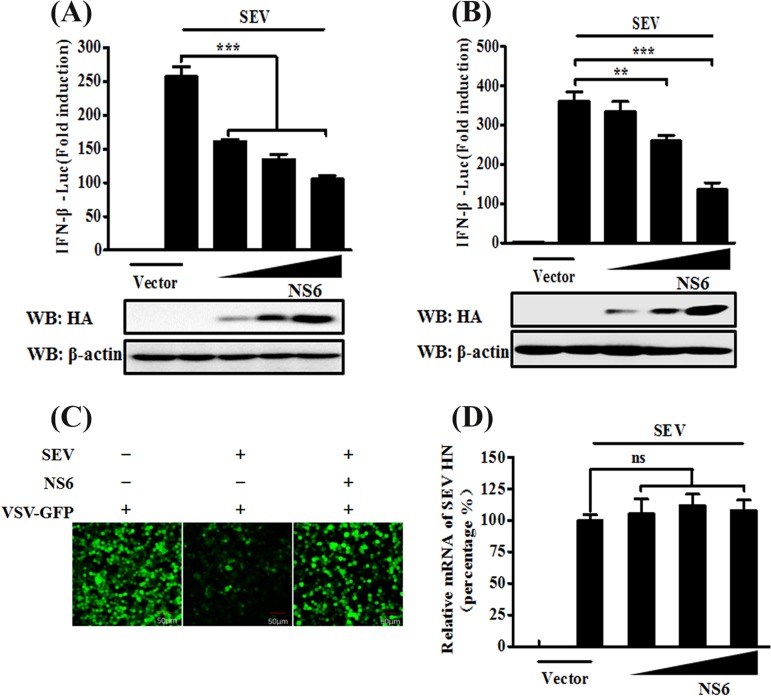
FIG 1.
NS6 inhibits SeV-mediated IFN-β production. (A and B) HEK-293T cells (A) or LLC-PK1 cells (B) cultured in 24-well plates were transfected with IFN-β-Luc plasmid and pRL-TK plasmid, together with increasing amounts (0.2, 0.4, or 0.8 μg) of plasmid pCAGGS-HA-NS6. At 24 h after transfection, cells were left untreated or were infected with SeV (10 hemagglutination units/well). The cells were then subjected to Dual-Luciferase assays at 12 h postinfection. The expression of PDCoV NS6 protein was confirmed by Western blotting with an anti-HA antibody. β-Actin served as a protein loading control. (C) HEK-293T cells were transfected with the indicated amounts of pCAGGS-HA-NS6 or empty vector. At 24 h after transfection, the cells were infected with SeV for 12 h, after which cell supernatants were harvested. Following UV irradiation, the harvested cell supernatants were overlaid onto fresh HEK-293T cells in 24-well plates. At 24 h after treatment, the cells were infected with VSV-GFP, and 12 h postinfection, virus replication was detected via fluorescence microscopy. (D) HEK-293T cells grown in 24-well plates were transfected with increasing quantities of pCAGGS-HA-NS6 or corresponding amounts of empty vector. At 24 h after transfection, cells were infected with SeV for 12 h. The total RNA was then extracted, and the SeV HN gene expression level was analyzed via quantitative real-time RT-PCR with normalization to the GAPDH gene expression level. The results shown are representative of data from three independent experiments: **, P < 0.01; ***, P < 0.001; ns, nonsignificant differences in data.