Figure 3.
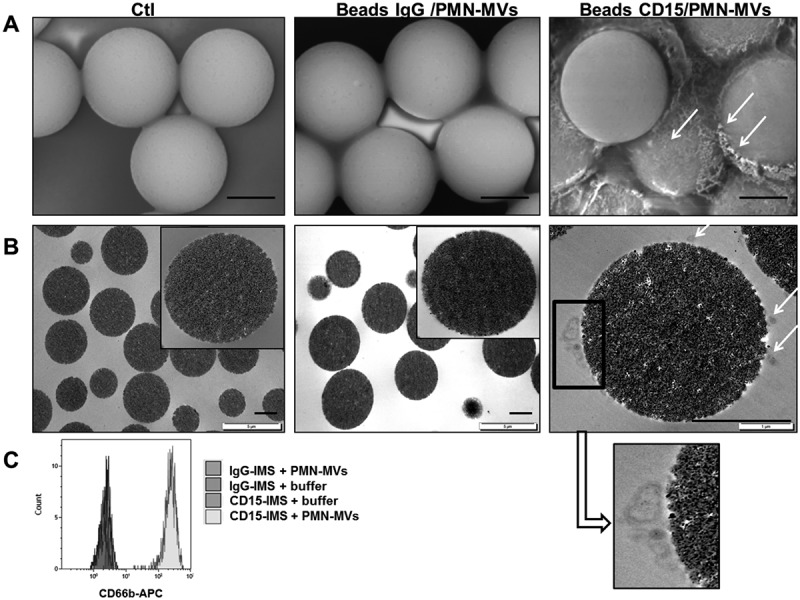
Microvesicle-dependent plasmin specificity.
A–B. Purified PMN-MVs spiked in MVFP were characterized after an immunomagnetic separation using magnetic beads coated with CD15 (CD15-IMS) or an irrelevant antibody (IgG-IMS) using scanning electron microscopy (A) and transmission electron microscopy (B) Ctl: Control condition was represented by bead-CD15 with buffer HEPES/BA. C. The presence of PMN-MVs on the surface of different beads was confirmed by flow cytometry using CD66b+ labelling. Scale bar: 2 µm. D. Platelet-free plasma (PFP) was depleted from MVs by either 0.5% Triton treatment or 0.1 µm filtration or high speed centrifugation (HSC). MV depletion efficiency was checked by flow cytometry measuring the annexin V + (AnnV+ MVs) events. E. The plasmin generation capacity (MV-PGC) using the immunomagnetic separation method was compared before and after MV depletion. n = 3. F. Different fibrinolytic enzymes were tested with the chromogenic substrate: uPA (25 × 10−2 UI/mL), t-PA (25 × 10−2 UI/mL), elastase (elast; 42 × 10−2 UI/mL), thrombin (0.5–32 × 10−2 UI/mL) and plasmin (0.5–35 × 10−2 UI/mL), Ctl: negative control was represented by PBS/BA buffer. G. The respective contribution of plasminogen activation and urokinase activity in the plasmin-dependent chromogenic test was evaluated using control samples (PFP enriched with LMVs). MVs captured by the CD15-beads were incubated in the presence or absence of different inhibitors of plasmin (alpha2-antiplasmin (α2AP), 275 nM), thrombin (hirudin, 10 U/mL), uPA (anti-uPA antibody, 25 µg/mL) or neutrophil elastase (anti-neutrophil elastase antibody, 10µg/mL) or an isotype control; Ctl = MPFP; n = 3. *** p ≤ 0.001, NS = p > 0.5.
