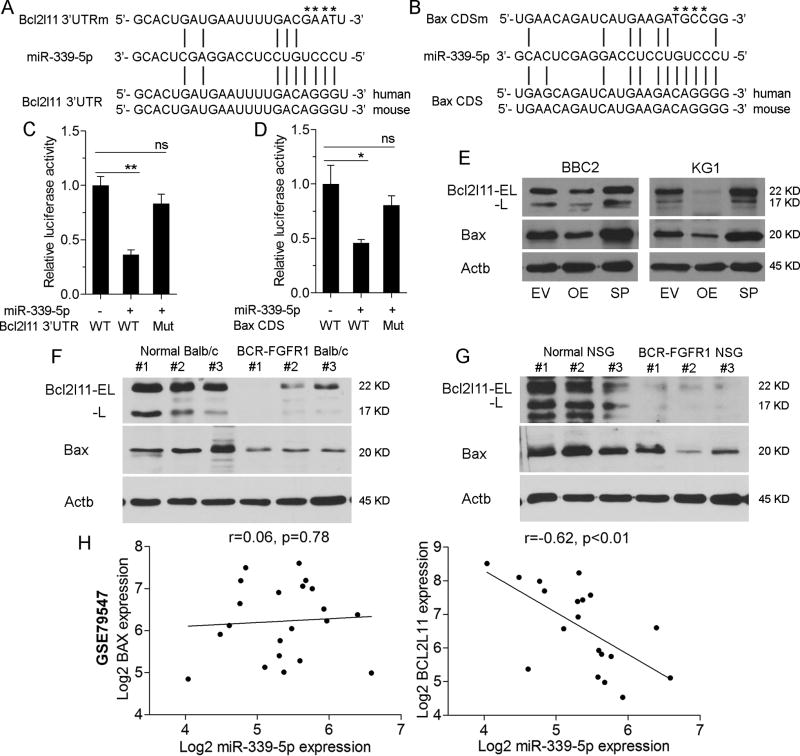
Figure 5. miR339-5p targets BCL2L11 and BAX.
miR339-5p target sites within the 3’UTR of BCL2L11 (A) and the coding region of BAX (B) were identified using prediction algorithms. In each case mutations of specific bases (shown by * above the sequence) in the binding regions were generated. In luciferase reporter assays (C) co-expression of miR-339-5p in cells carrying the wild type (WT) miR-339-5p target site in BCL2L11 shows a significant reduction in activation compared with a construct in which BCL2L11 sites have been mutated. The same relationship is observed in luciferase expression assays where the miR-339-5p target and mutated sequences for BAX are expressed (D). In BBC2 and KG1 cells, overexpression (OE) of miR-339-5p led to reduced expression levels of BCL2L11 (isoforms; EL = extra long and L = long) and BAX but co-expression of sponges (SP) targeting miR-339-5p prevents the suppressive effects on these two genes (E). In the murine SCLL model of BCF-FGFR (F), expression levels of BCL2L11 and BAX are reduced compared with splenic cells from normal mice. The same reduction in BCL2L11 and BAX expression levels is seen in primary AML cells derived from human cell SCLL that have been developed in immunocompromised (NSG) mice (G). Analysis of the pre-B-precursor ALL (GSE79547) data set, shows no change in relative levels of BAX expression compared to miR-339-5p expression (H) but a highly significant proportional decrease in BCL2L11 expression with increasing miR-339-5p expression. * p = <0.05, ** p = <0.01, ns = not significant.