Analysis of carbonic anhydrase mutants in Zea mays revealed slowed stomatal closure to high CO2 and dark transition as well as a role for carbonic anhydrase in water-use efficiency.
Abstract
Stomata regulate transpirational water loss and CO2 uptake for photosynthesis in response to changing environmental conditions. Research investigating stomatal movement has mostly been conducted in C3 eudicot species, which have very different CO2 requirements for photosynthesis relative to C4 grasses. Carbonic anhydrase (CA) catalyzes the hydration of CO2, and its activity has been linked to stomatal aperture regulation in eudicots. The number of Ca genes and their evolutionary history differ between monocots and dicots, and many questions remain unanswered about potential neofunctionalization and subfunctionalization of grass Ca paralogs and their roles in photosynthesis and stomatal conductance. To investigate the roles of different Ca genes in maize (Zea mays), we examined stomatal responses in ca1 and ca2 single mutants as well as a ca1ca2 double mutant. The ca1 and ca2 single mutants had 10% and 87% of the CA activity exhibited by the wild type, respectively, while ca1ca2 had less than 5% of wild-type CA activity. The ca mutants had higher stomatal conductance than the wild type and slower stomatal closure in response to increases in CO2 partial pressure. Contrary to previous reports in eudicots, ca mutants showed slowed stomatal closure in response to the light-dark transition and did not show differences in stomatal density compared with the wild type. These results implicate CA-mediated signaling in the control of stomatal movement but not stomatal development. Drought experiments with ca1ca2 mutant plants suggest a role for CA in water-use efficiency and reveal that Z. mays is not optimized for water-use efficiency under well-watered conditions.
In C4 photosynthesis, the enzyme carbonic anhydrase (CA) hydrates CO2 to form bicarbonate, which is the first catalyzed step in the C4 photosynthetic pathway (Badger, 1994). Bicarbonate and phosphoenolpyruvate are converted into oxaloacetic acid by phosphoenolpyruvate carboxylase (PEPC), the primary carboxylating enzyme of C4 plants. This C4 acid is used in the carbon-concentrating mechanism that allows C4 species to have reduced stomatal aperture while maintaining carbon flux for photosynthesis (Sage, 2004). The reduced stomatal aperture also decreases transpirational water loss relative to rates of photosynthesis and increases water-use efficiency in C4 relative to C3 plants (Ehleringer and Monson, 1993). Because the carbon-concentrating mechanism of C4 species enables a lower intercellular CO2 concentration compared with C3 plants with typically greater photosynthetic efficiency (von Caemmerer, 2000), it is possible that stomata of C4 plants are not as sensitive to changes in CO2 conditions. Thus, it is likely that stomatal CO2-sensing and -signaling mechanisms differ between grasses and other species or between C3 and C4 plants.
In addition to its role in C4 photosynthesis, CA also is involved in several cellular processes, including the generation of bicarbonate as a substrate for acetyl-CoA carboxylase during lipid metabolism, amino acid biosynthesis under low-CO2 conditions, and stomatal regulation (Hoang and Chapman, 2002; Hu et al., 2010; DiMario et al., 2016). Therefore, a detailed characterization of each isoform of CA may provide evidence of specialization and functional redundancies. In Arabidopsis (Arabidopsis thaliana), specific CA isoforms have been identified as components of the CO2 signaling pathway (Hu et al., 2010). βca1ca4 double mutant plants have higher stomatal conductance (gs) compared with wild-type plants (Hu et al., 2010), although neither single mutant displayed a phenotype, suggesting functional redundancies. In addition, although high CO2 levels trigger stomatal closure in ca1ca4 double mutant plants, the response is slower compared with the wild type. Despite these defects in the response to CO2, gs appears normal in response to abscisic acid and light treatments, suggesting that the role of CA is specific to a CO2 signaling pathway. The CO2 signaling pathway is particularly relevant because of the vital balance between CO2 uptake and transpirational water loss. Furthermore, global atmospheric CO2 increases coupled with changing climate conditions has the potential to perturb adaptive mechanisms needed for optimum growth (Ainsworth and Rogers, 2007). Although CA has been linked to CO2 signaling in the C3 eudicot Arabidopsis, it is currently unknown what role CA plays in stomatal regulation in C4 monocots.
The total CA activity found in leaves varies greatly between C4 species (Gillon and Yakir, 2000), as does the requirement for CA. In the C4 eudicot Flaveria bidentis, the first-order rate constant of CA (kCA) is 73.96 μmol m−2 s−1 Pa−1, and ca mutants with activities of 4.8 μmol m−2 s−1 Pa−1 require high CO2 concentrations for survival (von Caemmerer et al., 2004). However, in the C4 monocot maize (Zea mays), ca mutants have only a minor growth reduction at ambient CO2 (Studer et al., 2014), despite the low kCA (1.3 μmol m−2 s−1 Pa−1) of mutant plants compared with wild-type plants (kCA = 61 μmol m−2 s−1 Pa−1). In the C4 grass green foxtail (Setaria viridis), ca mutant plants with kCA = 8 μmol m−2 s−1 Pa−1 showed photosynthetic limitation at low CO2, similar to what was observed in Z. mays (Osborn et al., 2017). Although the ca mutants in Z. mays lack a pronounced phenotype at ambient CO2 levels, a metabolomics study in a diversity panel of Z. mays identified significant associations between single-nucleotide polymorphisms in genes encoding CA and metabolites related to photosynthesis (Zhang et al., 2015). Thus, while CA does not appear to be rate limiting for photosynthesis in Z. mays at current ambient CO2 levels, the overabundance of CA suggests that some conditions may favor high CA levels.
Here, we describe the functional characterization of the most abundantly expressed CA genes in Z. mays leaves, Ca1 and Ca2. The Ac/Ds transposable elements were used to generate ca1 single and ca1ca2 double mutants of these tandemly duplicated genes in Z. mays, and initial characterizations of the mutants were described previously (Studer et al., 2014). To fully understand the function of each gene copy, however, we generated and characterized both ca1 and ca2 single mutants. A screen was used to identify recombination events that generated ca1 and ca2 single mutant alleles, enabling a more refined genetic analysis of CA function. We utilized gas-exchange measurements of single and double ca mutant plants to monitor stomatal responses to changes in CO2 and light conditions. These measurements were then compared with the morphological and biochemical attributes of the leaf. Furthermore, Z. mays ca1ca2 double mutant plants were grown under several watering regimes to monitor water use and define a potential role for CA in drought responses. Our results show that altering CA function affects water-use efficiency in Z. mays.
RESULTS
Generation of ca2 Single Mutant Plants
The ca1 and ca2 single mutant plants used in this study were obtained by screening a segregating population containing the ca1ca2 alleles described previously (Studer et al., 2014). Three recombinant plants were recovered: one having a frame-shift mutation in ca1 with a wild-type allele of Ca2, and the other two containing the Ds insertion in ca2 without the presence of the frame-shift mutation in Ca1. This recombination frequency indicates that the ca1 and ca2 genes are located 1.6 cM apart. The newly generated ca1 footprint mutant allele is an independent line from the two previously characterized ca1 alleles and was caused by an imprecise Ds excision that left an 8-bp insertion and changed the frame of the predicted protein. The newly recovered ca2 single mutant plants carry the original Ds insertions; therefore, they are stable in the absence of Ac transposase.
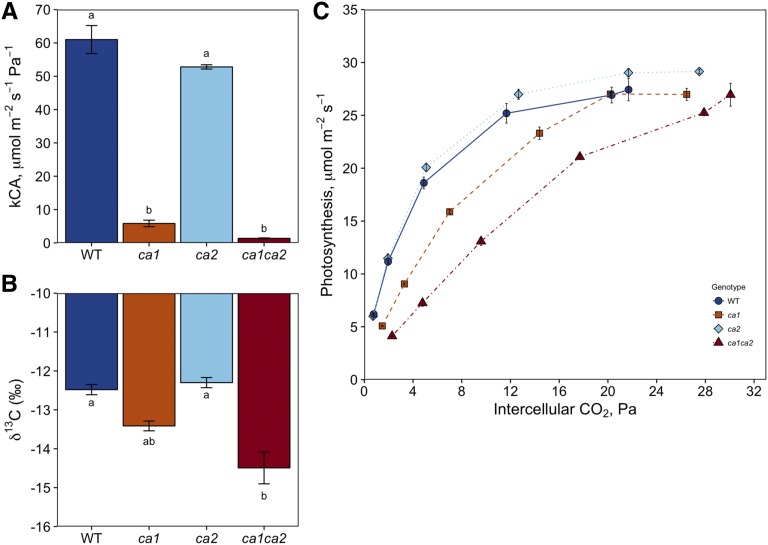
Consistent with previous reports, ca1 mutants had 10% of the total leaf CA observed in wild-type plants, and the ca1ca2 mutant plants only had 2% of wild-type CA levels (Fig. 1). The newly generated ca2 single mutant plants had 87% of wild-type levels of total leaf CA. The measurements of CA levels in each single mutant and the ca1ca2 double mutant show the additive nature of CA1 and CA2 in the leaf. This result confirms the observation that, while Ca2 contributes 22.6% to the CA transcript pool in the leaf (Studer et al., 2014), it makes up only 13% of the total CA activity. The A/Ci measurements were consistent with kCA and showed that ca1 single and ca1ca2 double mutant plants had significant differences in the rates of photosynthesis at low CO2, but the rates in ca2 single mutant plants were nearly identical to those in the wild type (Fig. 1). However, only the loss of both CA1 and CA2 activities, but not CA1 or CA2 alone, resulted in a significantly lower carbon isotope signature of the leaf compared with wild-type plants (Fig. 1).
Figure 1.
CA activity and stable isotope composition in ca mutant plants. A, CA activity expressed as kCA in ca1, ca2, and ca1ca2 plants compared with wild-type (WT) plants. B, Stable isotope composition (δ13C) measured in wild-type and mutant leaves. Different lowercase letters denote significant differences between groups (Tukey’s posthoc test, P < 0.05). C, Net rate of CO2 assimilation response to intercellular CO2 partial pressure (A/Ci curves) for each genotype measured at PPFD = 1,500 μmol m−2 s−1 and leaf temperature of 25°C. Data are presented as averages of three to four individuals ± se.
Phenotypic Characterization
The ca mutants were further characterized to examine the consequences of a disruption in CA activity on photosynthetic enzyme activities, biomass accumulation, and stomatal density (Table I). While there was a significant difference in CA activity between genotypes (P < 0.0001), no differences were observed between genotypes for total leaf PEPC and Rubisco activity (P = 0.5232 and 0.6397, respectively). Furthermore, while significant differences in net photosynthetic rate (A) were observed between wild-type and ca1ca2 mutant plants, there was no significant difference in biomass between genotypes in fresh and dry weight measures (P = 0.0674 and 0.1449, respectively). Unlike the βca1ca4 double mutants in Arabidopsis (Engineer et al., 2014), none of the ca mutants in Z. mays had altered stomatal density (P = 0.4261).
Table I. Biochemical and physiological characteristics of CA mutants.
Data are presented as averages ± se. No significant differences were observed between genotypes.
| Genotype | PEPC | Rubisco | Fresh Weight | Dry Weight | Stomatal Density |
|---|---|---|---|---|---|
| μmol m−2 s−1 | g | ||||
| Wild type | 277.5 ± 14.8 | 31.5 ± 1.4 | 215.6 ± 6.9 | 22.4 ± 1.4 | 49.6 ± 2.1 |
| ca1 | 268.1 ± 31.4 | 34.4 ± 5.1 | 211.8 ± 14.6 | 21.1 ± 1.9 | 50.5 ± 0.5 |
| ca2 | 269.9 ± 31.9 | 31.7 ± 1.8 | 209.2 ± 14.8 | 22.7 ± 3.2 | 45.8 ± 1.4 |
| ca1ca2 | 307.6 ± 8.7 | 35.3 ± 1.6 | 172.9 ± 11.1 | 17.0 ± 1.0 | 48.0 ± 2.3 |
Steady-State Measurements
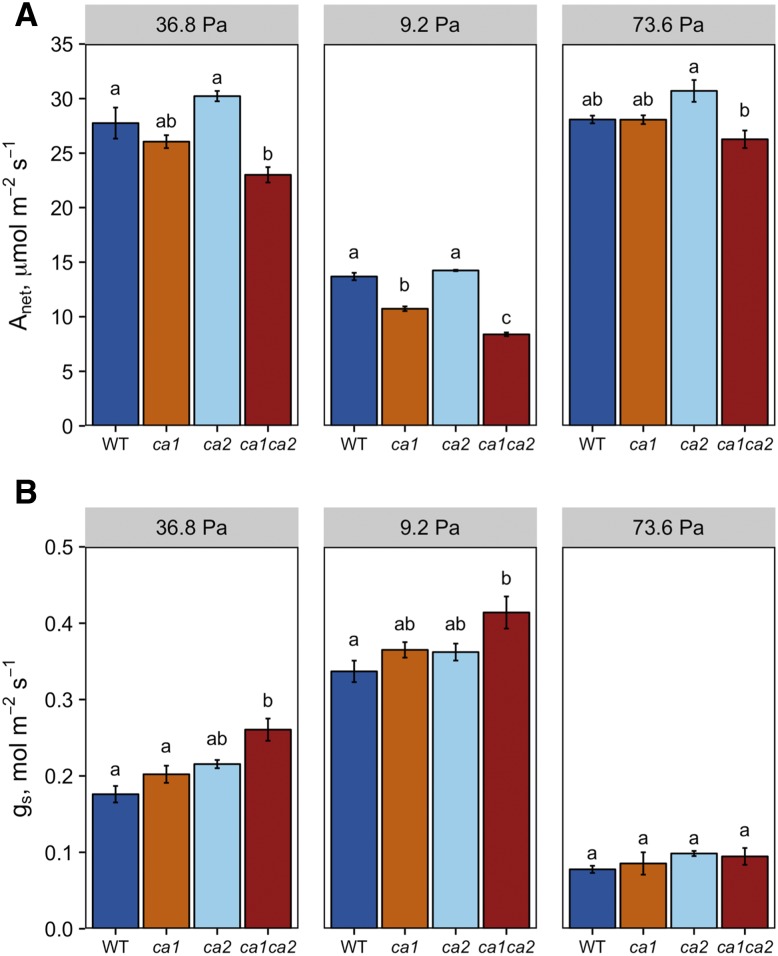
Transient rates of A and gs were measured every 60 s as CO2 partial pressure transitioned between 36.8 and 9.2 Pa (400 and 100 µL L−1) for 30 min each and 60 min at 73.6 Pa (800 µL L−1; Fig. 2). To minimize the confounding effects on A and gs, the leaf temperature, concentration of water in the sample cuvette, and vapor pressure deficit (VPD) between the leaf and atmosphere were held constant (Supplemental Fig. S1). Steady-state conditions were calculated from the last 10 min under each condition.
Figure 2.
Steady-state photosynthesis and gs measurements during the CO2 response. Steady-state measurements of photosynthesis (A) and gs (B) were calculated from the last 10 points at each CO2 partial pressure. Bars represent averages of three to four individuals ± se. Leaf temperature (25°C) and PPFD (1,500 μmol m−2 s−1) were held constant in the leaf chamber. Different lowercase letters represent significant differences between groups (Tukey’s posthoc test, P < 0.05). WT, Wild type.
At ambient CO2 levels (36.8 Pa), only the ca1ca2 double mutant showed significantly lower steady-state A compared with wild-type plants (P = 0.019; Fig. 2A). In addition, ca2 mutants had significantly higher A than ca1ca2 mutant plants (P = 0.002). The effect of the CA mutations became more evident at 9.2 Pa CO2, when significant differences in A were observed for all genotypes except between wild-type and ca2 mutant plants (P = 0.440). Differences in A were minimized at 73.6 Pa. The average steady-state A for ca2 mutants was consistently higher than for wild-type plants across all CO2 conditions; however, this trend was not statistically significant (36.8 Pa, P = 0.344; 9.2 Pa, P = 0.44; and 73.6 Pa, P = 0.174). Similar increases in gs were observed in ca1 and ca2 single mutants at 36.8 Pa compared with wild-type plants. An additive increase in gs was observed in ca1ca2 double mutant plants (Fig. 2B).
CO2 and Light Response
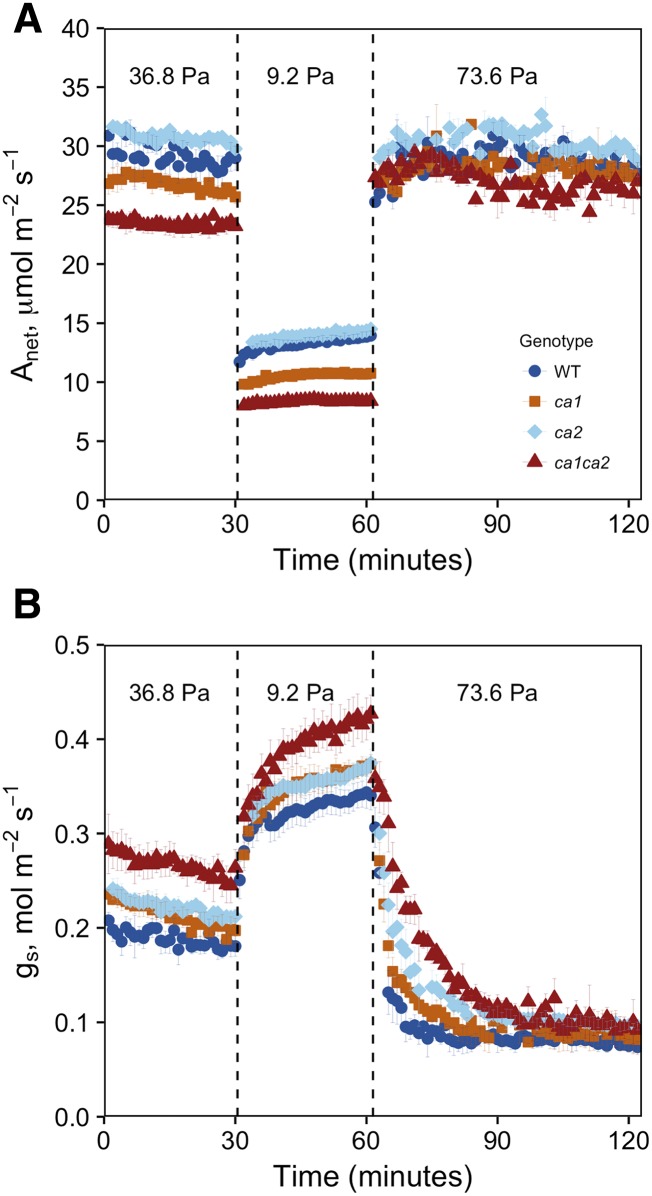
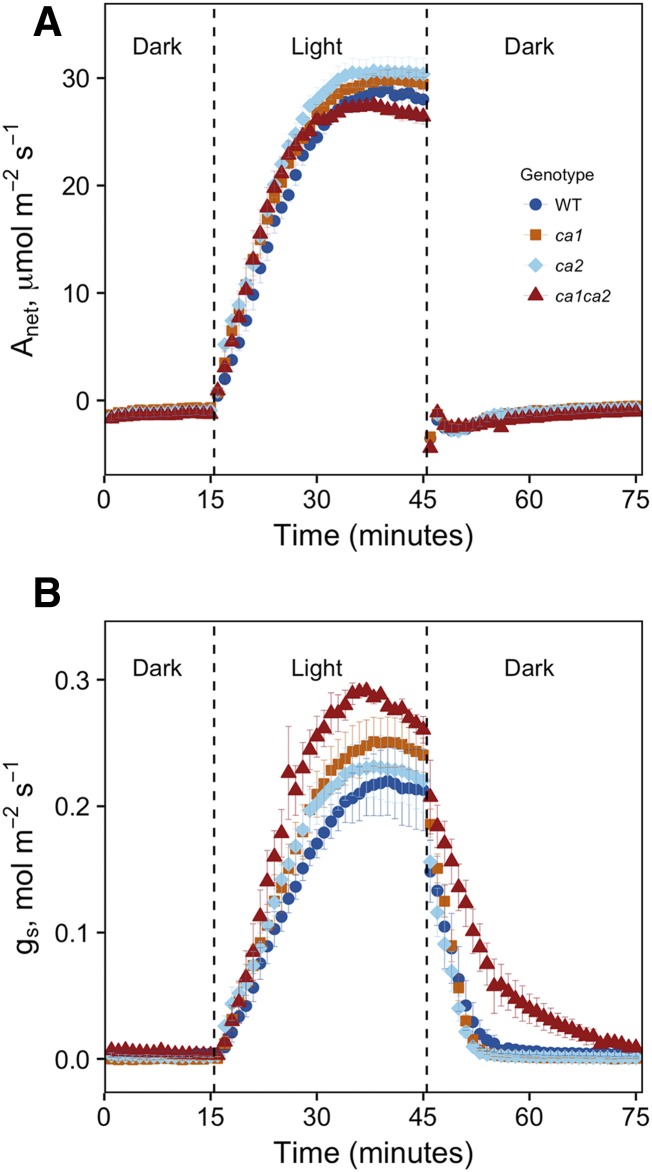
In addition to steady-state differences in gs, significant differences in rates of stomatal closure also were observed between genotypes (Fig. 3; Table II). These differences were tested by calculating the time to 90% of the steady-state open or closed value. Stomatal closure was slower in ca1ca2 double mutants compared with wild-type and ca1 single mutant plants when the CO2 was raised to 73.6 Pa (P = 0.003 and 0.0497, respectively), but it was not significantly slower than in ca2 mutants (P = 0.06). Both ca1 and ca2 single mutants showed slowed stomatal closure following a transition from low to high CO2 (Fig. 3), but they were not significantly slower than wild-type plants. Interestingly, ca1ca2 double mutant plants also showed slowed closure when transitioned from light to dark (Table II; Fig. 4). No difference in the rate of stomatal closing was observed in the single mutant plants compared with the wild type in response to light-dark transitions. In addition, no difference in the rate of stomatal opening was observed in response to CO2 or light (Table II).
Figure 3.
Gas-exchange measurements during the CO2 response. Photosynthesis (A) and gs (B) were measured in response to CO2 in wild-type (WT) and mutant plants. Points represent averages of three to four individuals ± se. Leaf temperature (25°C) and PPFD (1,500 μmol m−2 s−1) were held constant in the leaf chamber. Leaf-to-air VPD was maintained between 1.3 and 1.5 kPa across all conditions by manually controlling water concentration in the leaf chamber (∼20 mmol mol−1; Supplemental Fig. S1).
Table II. Rates of stomatal response.
Data are presented as averages ± se. Values represent time (min) to 90% open or closed response.
| Genotype | CO2 Opena | CO2 Closeb | PAR Opena | PAR Closeb |
|---|---|---|---|---|
| Wild type | 13 ± 2 | 6 ± 2 a | 18 ± 1 | 7 ± 1 a |
| ca1 | 13 ± 2 | 14 ± 4 a | 17 ± 1 | 7 ± <1 a |
| ca2 | 10 ± 2 | 15 ± 5 a,b | 16 ± 1 | 6 ± <1 a |
| ca1ca2 | 14 ± 2 | 27 ± 2 b | 15 ± 2 | 16 ± 2 b |
No significant differences were observed between genotypes.
Lower case letters show significant differences between genotypes.
Figure 4.
Gas-exchange measurements during the light response. Photosynthesis (A) and gs (B) were measured in response to dark-light and light-dark transitions. Points represent averages of three to four individuals ± se. Leaves were clamped in an unilluminated LICOR chamber and allowed to acclimate. Lights were turned on (PPFD = 1,500 μmol m−2 s−1) for 30 min, then turned off to measure stomatal closure. Measurements were taken at constant CO2 in the leaf chamber (36.8 Pa) and leaf temperature (25°C). Leaf-to-air VPD was maintained around ∼1.5 kPa across all conditions by manually controlling water concentration in the sample (∼18–19 mmol mol−1). WT, Wild type.
Effect of Reduced CA on Drought Tolerance
Because ca1ca2 mutants showed the most striking phenotypes, with consistently lower A and higher gs than wild-type plants, double mutant plants were grown to assess the effect of reduced CA activity on drought tolerance. Wild-type and ca1ca2 mutant plants were grown in five different treatments: 100%, 80%, 60%, 40%, and 20% field capacity (FC), plus or minus the error associated with the plant weight over the course of the experiment. Plants were grown in pots in the greenhouse, and the water treatments were based on the FC of the soil. Whole-plant transpiration was calculated by weighing the pots daily and subtracting the average amount of water that evaporated from the empty pots from the total amount of water lost from each treatment pot. Surprisingly, no significant difference in whole-plant transpiration was observed between ca1ca2 mutant plants and wild-type plants in any of the treatments (Table III). However, statistically significant differences were observed for plant height and dry weight biomass between the two genotypes (Table III). Furthermore, the average percentage differences between wild-type and mutant plants for plant height and dry weight were similar across all treatments (∼12.3%). This resulted in a difference between wild-type and ca1ca2 mutant plants for water-use efficiency across all treatments (Fig. 5).
Table III. Drought stress phenotypes.
Data are presented as averages ± se. Days, Number of days under treatment conditions; N, replicates; E, transpiration. Asterisks indicate significance when comparing the wild type with the mutant using pairwise Student’s t test: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
| Genotype | Treatment | Days | N | E | Plant Height | Dry Weight |
|---|---|---|---|---|---|---|
| L | cm | g | ||||
| Wild type | 100% | 19 | 7 | 10.9 ± 0.4 | 120 ± 2.2 | 51.4 ± 1.6 |
| ca1ca2 | 100% | 19 | 7 | 11.4 ± 0.2 | 109 ± 2.6** | 45.6 ± 1.0* |
| Wild type | 80% | 19 | 8 | 9.6 ± 0.3 | 118 ± 1.6 | 51.2 ± 1.1 |
| ca1ca2 | 80% | 19 | 8 | 9.7 ± 0.2 | 105 ± 2.4*** | 44.6 ± 0.9*** |
| Wild type | 60% | 18 | 8 | 7.8 ± 0.2 | 103 ± 1.4 | 46.9 ± 1.0 |
| ca1ca2 | 60% | 18 | 7 | 7.8 ± 0.1 | 86 ± 2.3*** | 40.0 ± 0.7*** |
| Wild type | 40% | 16 | 8 | 4.6 ± 0.1 | 76 ± 2.6 | 34.0 ± 0.9 |
| ca1ca2 | 40% | 16 | 7 | 4.8 ± 0.4 | 66 ± 3.1* | 29.7 ± 1.4* |
| Wild type | 20% | 11 | 7 | 0.9 ± 0.2 | 61 ± 1.8 | 24.0 ± 0.8 |
| ca1ca2 | 20% | 11 | 7 | 1.1 ± 0.1 | 54 ± 1.0** | 21.2 ± 0.4* |
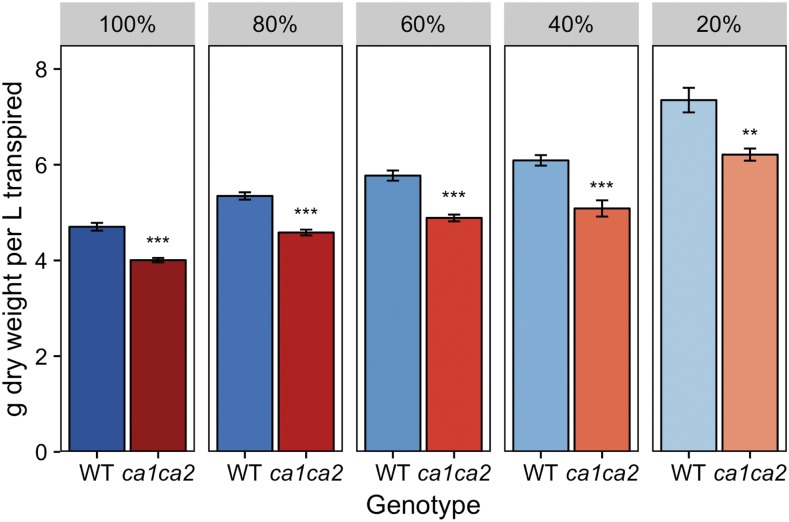
Figure 5.
Water-use efficiency of wild-type (WT) and ca1ca2 plants grown under different drought conditions. Dry weight biomass was measured at the end of the experiment. Water treatments were determined based on the water-holding capacity of the soil. Transpiration was calculated over the course of the treatments by weighing each pot daily and controlling for evaporation using empty control pots. Data are presented as averages of seven to eight individuals ± se. **, P < 0.01 and ***, P < 0.001.
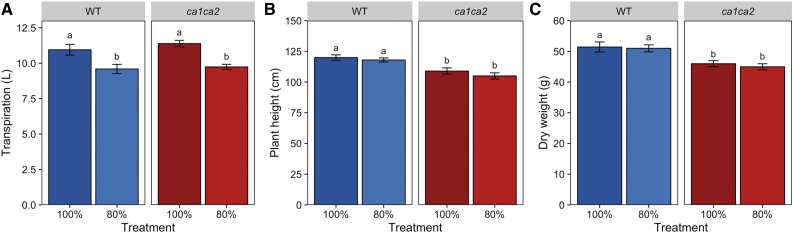
With these data, we were also able to investigate the water requirement for normal plant growth in this experiment. There was no difference in plant growth between wild-type plants given 100% and 80% FC (Fig. 6). Thus, 80% of the pot’s water-holding capacity is sufficient for normal growth. However, plants under 100% FC conditions transpired significantly more water (∼10%; P = 0.016) over the course of the experiment than plants grown at 80% FC to achieve similar growth (Fig. 6). An identical trend was seen for ca1ca2 mutant plants, suggesting that Z. mays plants do not operate at their maximum water-use efficiency under FC conditions. This is also supported by the observed trend across treatments for the amount of water needed to produce 1 g of dry weight biomass. Under 100% FC conditions, 213 mL of water was required, but under 20% FC conditions, only 137 mL of water was used (Fig. 5).
Figure 6.
Transpirational water loss compared with plant growth of wild-type (WT) and ca1ca2 plants under well-watered conditions. Transpiration (A), plant height (B), and dry weight (C) are shown for plants grown under 100% and 80% FC. Water treatments were determined based on the water-holding capacity of the soil. Data are presented as averages of seven to eight individuals ± se. Different lowercase letters represent significant differences between groups (pairwise Student’s t test, P < 0.05).
DISCUSSION
CA1 and CA2 account for more than 95% of the total CA activity in Z. mays leaves (Studer et al., 2014). Although stomatal movement differences were observed in ca single mutants (Fig. 3), a double ca mutant was necessary to observe significant gas-exchange differences compared with wild-type Z. mays under standard growth conditions. This result is likely due to the presence of multiple CA isoforms with redundant function in the same cell type. Based on these results, we hypothesize that the tandemly duplicated Ca genes are at least partially subfunctionalized with respect to their roles in stomatal movement, photosynthetic rates, and carbon isotope composition.
In Arabidopsis and rice (Oryza sativa) ca mutants, stomatal closure defects are only observed in response to elevated CO2, suggesting that the role of CA is specific to the CO2 signaling pathway (Hu et al., 2010; Chen et al., 2017). However, Z. mays ca mutant plants exhibit slower stomatal closure in response to both high CO2 and light-dark transitions. This result indicates that, unlike in Arabidopsis and rice, CA may function in stomatal closure in response to light or in stomatal movement downstream of the convergence point between the CO2 and light signaling pathways. It is interesting that stomata of the ca1ca2 double mutant opened rapidly in response to light, and then conductance decreased after reaching a maximum (Fig. 4). We hypothesize that rapid stomatal opening after exposure to light cannot be modulated properly in the ca1ca2 mutant plants because of slowed stomatal closure. Thus, the plants initially increase gs too much and need time to reach the appropriate steady-state gs.
The role of Ci as a signal in stomatal responses to light has been debated in the literature: low Ci has been suggested to increase gs, and high Ci would reduce gs (Lawson et al., 2014). Furthermore, some have speculated that light-to-dark stomatal responses may be mediated by an increase in Ci as photosynthesis decreases and respiration increases (Roelfsema et al., 2002; Hashimoto et al., 2006). However, the data presented here are not consistent with Ci as a signal for stomatal closure. For example, the ca mutants tended to have higher Ci than the wild type due to decreased photosynthetic rate and increased gs (Supplemental Fig. S2). Therefore, if stomatal closure in the dark is driven by elevated Ci, then the ca1ca2 mutants would be expected to have a more rapid stomatal closure.
While Arabidopsis ca mutants have alterations in both stomatal density and aperture, there is no difference in stomatal density in Z. mays ca mutants. The lack of stomatal density differences also was observed recently in ca mutants in rice (Chen et al., 2017). This discrepancy may be indicative of different developmental trajectories in monocots and dicots: the patterning of stomatal density in eudicots occurs later in leaf development and is more subject to environmental perturbation, whereas patterning of stomatal density occurs earlier in leaf development in monocots and is less sensitive to environmental cues. Indeed, many of the known stomatal patterning genes are expressed in a narrow developmental window at the base of a developing leaf blade in both Z. mays and rice (Li et al., 2010; Wang et al., 2014). This apparent insensitivity to short-term environmental variability suggests that grasses are more dependent on the regulation of stomatal aperture to control gs.
The ca2 single mutant detailed here provides some insights into the role of CA2 in Z. mays. The discrepancy between the transcript levels and the enzyme activity of the CA isoforms suggests posttranscriptional differences between Ca1 and Ca2 transcripts or differences in the kinetic properties of the enzyme isoforms. Furthermore, the increase in gs observed in ca2 mutant plants was not proportional to the CA2 contribution to the total CA activity, suggesting that it may be concentrated in a specific cellular or subcellular location that maximizes its role in the CO2 response. Although we consistently observed higher A and gs in ca2 mutants under all CO2 conditions, differences between ca2 mutant and wild-type plants were not statistically significant with respect to A, gs, and A/gs even under low-CO2 conditions. These results suggest that the 13% reduction in total leaf CA observed in the ca2 mutant is not sufficient to limit A, even under low CO2.
Based on our results, we suggest a model for the subfunctionalization of the duplicated Ca2 gene copy. We speculate that the ancestral gene that encodes CA1 has both stomatal signaling and photosynthetic functions, whereas the newly duplicated CA2 copy may have a specialized function in guard cells. From our data, we cannot conclusively determine the extent to which CA2 functions in photosynthesis. It is clear that, when CA1 is mutated, CA2 has the ability to provide bicarbonate for photosynthesis and, thus, is not present exclusively in guard cells. However, because there is no indication of a decrease in A in ca2 single mutants, this could suggest that the difference between ca1 and ca1ca2 mutant plants is the extent to which CA2 is redundant. From this work, we are unable to determine the cellular or subcellular localization of these CA isoforms. Because both CA1 and CA2 can contribute bicarbonate for photosynthesis at some level, this would suggest that, at least in mesophyll cells, these proteins are located in the cytosol. However, it remains unknown if either isoform is localized to the plasma membrane or in chloroplasts, as seen in Arabidopsis (Fabre et al., 2007; Hu et al., 2015).
Although a significant difference in gs was observed in ca1ca2 double mutants in Z. mays, no differences in gs were observed in S. viridis ca mutants (Osborn et al., 2017). One possible explanation for this result may be that Z. mays and S. viridis, which have independent origins of C4 photosynthesis, have different requirements for CA activity in the leaf even though they have similar leaf CA rate constants (S. viridis kCA = 61–84 μmol m−2 s−1 Pa−1; Osborn et al., 2017). Alternatively, the impact on gs may relate to the severity of the ca knockdowns. The most dramatic knockdowns in S. viridis have kCA = 8 μmol m−2 s−1 Pa−1, which is approximately 6 times more activity than what was observed in the Z. mays ca1ca2 double mutants (kCA = 1.3 μmol m−2 s−1 Pa−1). Another possibility is that there is a CA isoform involved in stomatal signaling that was not affected by the knockdown construct used in S. viridis. This is quite possible given that there are three tandem Ca genes in S. viridis and no clear ortholog to Ca2 (Studer et al., 2016).
Higher gs in ca mutant plants would seem to indicate a higher whole-plant transpirational loss, which would be detrimental under water-limiting conditions. However, the difference in gs between mutant and wild-type plants did not translate into a significant difference in water losses due to whole-plant transpiration. It is possible that the reduction in biomass, and therefore the reduced surface area for transpiration, balances the difference in gs. It is also possible that the high-temperature and low-humidity conditions under which the drought experiment was conducted increased VPD to the point that the signal to close stomata through the abscisic acid pathway overrode the signal to open stomata in response to low Ci. This would indicate that Z. mays is more sensitive to high VPD stress than to intercellular CO2 limitations, which is consistent with correlations between climate change and Z. mays yield losses in the Midwest (Lobell et al., 2014). Regardless, the difference in water-use efficiency (Fig. 5) between wild-type and mutant plants indicates a limitation for photosynthesis that resulted in a reduction in biomass.
An important observation in the drought experiment was a significant difference in transpiration rates of wild-type plants under 100% and 80% FC conditions. It was not surprising that plants grown at 100% and 80% FC would be morphologically similar and produce the same amount of biomass. This observation indicates that water is not limiting for growth at 80% FC. However, it is intriguing that plants grown at 100% FC used 10% more water to achieve the same growth as plants grown at 80% FC. This result indicates that, under these growth conditions, Z. mays is not water-use efficient when well watered. This provides a potential target for crop improvement and merits testing under field conditions. Conserving water under well-watered conditions would build up water reserves in the soil that could be used later in the season during dry periods. While most studies look at water-use efficiency under drought conditions, further investigation into the water-use efficiency under well-watered conditions could provide insight into other ways of optimizing water use in Z. mays.
In the experiments presented here, ca1ca2 mutant plants tended to produce less biomass than wild-type plants, consistent with previous measurements (Studer et al., 2014). However, this difference is only significant for plants grown for the drought experiment, which were 6.5 weeks old, compared with 4-week-old plants grown for the stomatal response experiments. Very low CA activity likely has a small but cumulative effect on photosynthesis and, thus, would be more evident toward the end of the plant’s life cycle. While these results highlight the relatively subtle effects of CA on plant performance, CA function may be important for achieving optimum efficiency over an entire growing season. This function may help explain the large amount of variation in CA activity observed between species (Gillon and Yakir, 2000). If levels of CA correlated with the sensitivity of stomata, variable conditions, such as those experienced in a field environment, may require CA to fine-tune stomatal responses in a dynamically changing environment.
CONCLUSION
Characterization of the newly generated ca mutants has enabled us to investigate the contribution of specific CA genes to photosynthesis and stomatal movement. Our data show that, in Z. mays, CA plays a role in stomatal movement in response to CO2 and light; however, it does not affect stomatal patterning. Differences observed between the ca1 and ca2 single mutants lead us to speculate that CA1 could be the main isoform providing bicarbonate for C4 photosynthesis, while CA2 may be more specialized and required for normal guard cell movement. Drought experiments revealed that ca1ca2 double mutants did not transpire more than the wild type but had reduced biomass, presumably due to a limitation of photosynthesis. Collectively, these results indicate that there is a benefit to high levels of CA, which enables plants to maintain high rates of photosynthesis under CO2-limiting conditions and to fine-tune stomatal movements to changing environmental conditions.
MATERIALS AND METHODS
Screen for ca2 Single Mutant Alleles
Maize (Zea mays) plants heterozygous for the cis ca1ca2 mutations (Studer et al., 2014) were self-pollinated and produced progeny that segregated 1:2:1 for the mutated ca locus. From this ear, 192 kernels were planted, and DNA was extracted from each seedling using a CTAB DNA extraction protocol. An approximately 2.5-cm2 piece of leaf tissue was harvested from the youngest leaf of 8- to 11-d-old Z. mays seedlings. Tissue samples were placed in 96 plastic racks (Fisher Scientific, catalog no. 50-823-921) of 1.4-mL tubes (Fisher Scientific, catalog no. 50-823-825) containing two one-eighth-inch Grade 1000 Type 430 Stainless Steel Balls (Abbott Ball) on ice. Tubes were capped with eight-well strip caps (VWR, catalog no. 82006-694). After collection, liquid nitrogen was used to freeze the plates containing the tissue. The tissue was homogenized using a paint shaker (Harbil, model no. 5G-HD) set to shake for 2 min. The plates were then removed and again placed in liquid nitrogen for 1 min. This was repeated until the tissue was fully homogenized (two to four rounds). One hundred milliliters of CTAB buffer was prepared by mixing 50 mL of water, 10 mL of 1 m Tris-HCl (pH 8), 2 g of CTAB, 0.2 g of sodium bicarbonate, 4 mL of 0.5 m EDTA (pH 8), and 28 mL of 5 m sodium chloride. Water was then added to the solution up to 99 mL, and 1 mL of β-mercaptoethanol was added immediately before use. The strip caps were removed with forceps, and 400 μL of CTAB buffer was added to each sample. The caps were replaced, and the plates were shaken by hand until the tissue was suspended in the CTAB buffer. The plates were spun in a centrifuge (Eppendorf, model no. 5410R) with a swinging-bucket rotor at 2,000 rpm for 2 min. The samples were then incubated for 30 min in a water bath at 65°C.
After incubation, the plates were removed and allowed to cool at room temperature for 10 min. The caps were removed and discarded. Then, 400 μL of chloroform:isoamyl alcohol (24:1) was added to each sample and mixed by pipetting up and down using wide-bore tips. A mat (Fisher Scientific, catalog no. 50-823-876) was used to cover the tubes, and the plates were centrifuged at 4,000 rpm for 15 min at room temperature. A total of 100 μL of the aqueous layer was removed from each sample and placed on a new plate (VWR, catalog no. 89049-178) containing 100 μL of ice-cold 100% isopropanol. The samples were mixed by pipetting up and down. The plate was then covered with a plastic seal (VWR, catalog no. 89091-702) and placed at 4°C for 1 h. After DNA precipitation, the plate was centrifuged at 4,000 rpm for 15 min at room temperature. The isopropanol was removed by carefully inverting the plate, and the DNA pellet was washed with 500 μL of ice-cold 70% (v/v) ethanol. The plate was again covered with a plastic seal and centrifuged at 4,000 rpm for 15 min at room temperature. The ethanol was removed by inverting the plate, and the DNA pellet was dried at 30°C using an Eppendorf Vacufuge for 10 min. The DNA pellet was resuspended in 100 μL of water and placed at 4°C overnight. DNA concentrations were determined using a Nanodrop 2000C spectrophotometer and then diluted for downstream applications. Individuals were tested for the presence of the Ds element in ca2 using standard PCR (genic-genic primers AJS007 [5′-GGCTGCTTATTGTCCCATGT-3′] and AJS097 [5′-CTGGAGTCTGGATATACCATTCC-3′]; genic-Ds primers AJS097 [5′-CTGGAGTCTGGATATACCATTCC-3′] and AJSR05 [5′-CGTCCCGCAAGTTAAATATGA-3′]) and visualized using agarose gel electrophoresis. The presence of the 8-bp Ds footprint in ca1 was detected based on the difference in melting temperatures (wild type = 80.7°C–81.7°C; mutant = 83.2°C–84.3°C) of the PCR product amplified by two genic primers (AJS405 [5′-AATGGGACCCTCAAGCTGAT-3′] and AJS406 [5′-TGTGAGGAACTCTCCTGAGACA-3′]) that span the 8-bp insertion. Fragment amplification and melt curves were produced by using LightCycler 480 SYBR Green I Master Mix and a Roche LightCycler 480 instrument following the manufacturer’s recommendations. Analysis of the melt curve was performed using the Tm calling option of the Roche software and manual checking.
Plant Growth Conditions
Seeds of wild-type, ca1, ca2, and ca1ca2 plants were planted in 2-gallon pots filled with LC-1 Sunshine mix (Sun Gro Horticulture) and grown in a greenhouse at Washington State University with 14-h days at 27°C and 10-h nights at 20°C, with supplemental lighting to provide a fluence of at least 600 W m−2. All plants were fertilized weekly (Peters 20-20-20), treated with a slow-release fertilizer (21-5-5), and supplemented with Spring 330 iron chelate (BASF).
Gas Exchange
Photosynthesis and gs were measured on the uppermost, fully expanded leaf of 4-week-old plants (leaf 5 or 6) using a LICOR-6400XT. Measurements were performed by controlling leaf temperature at 25°C and manually adjusting water concentrations to maintain a leaf-to-air VPD between 1 and 1.5 under all conditions. Each plant was acclimated to the measurement conditions for 15 to 30 min. Four plants were measured for the wild type and ca1ca2, and three plants were measured for ca1 and ca2 single mutants.
The stomatal response to CO2 was assessed by maintaining constant PPFD (1,500 μmol photons m−2 s−1) and controlling the CO2 concentration in the sample cuvette. Each plant was initially measured for 30 min at 36.8 Pa CO2. After 30 min, the CO2 partial pressure was decreased to 9.2 Pa, and measurements were taken every 1 min for 30 min. Finally, the CO2 concentration was increased to 73.6 Pa, and data were collected for 60 min. At the completion of these measurements, a short CO2 response curve was taken by controlling the CO2 partial pressure entering the cuvette at 73.6, 55.2, 36.8, 18.4, 9.2, and 4.6 Pa.
The stomatal response to light was assessed by maintaining a constant 36.8 Pa CO2 in the sample cuvette. Plants were acclimated in a dark cuvette. Once stomata had closed, measurements were taken every 1 min for 15 min. Then, PPFD was set to 1,500 μmol photons m−2 s−1, and data were logged every 1 min for 30 min. After 30 min, the light source was turned off and measurements were taken for 30 min to monitor the stomatal closure response.
Statistically significant differences in rates of stomatal opening and closing were assessed by calculating the time required for each plant to reach 90% of the open or closed response value for each condition. This method of calculating differences in response has been used to assess changes in gs in response to sunflecks (Leakey et al., 2005). The last conductance value measured prior to changing conditions was used as the starting value for each condition, and the steady-state open/closed value was determined by averaging the final 10 points in each condition. The point at which each plant crossed its threshold of 90% open/closed response was applied to a one-way ANOVA, and subsequent pairwise comparisons were performed using Tukey’s posthoc test.
Stomatal Density
Leaf material for measuring stomatal density was taken from the same portion of the leaf measured for gas exchange. Samples were immediately frozen in liquid nitrogen and stored at −80°C. Frozen leaf discs were used to measure stomatal density. A Nanofocus μsurf explorer optical topometer with a 20× lens and a 0.6 numerical aperture was used to capture 0.8-mm × 0.8-mm images of both the abaxial and adaxial leaf surfaces. Two images were taken per side per disc. Two to three leaf discs were measured per plant replicate, and three to four replicates were used for each genotype. Thus, stomatal density for a given genotype was calculated from a minimum of 24 images.
Carbon Isotope Composition
Samples for carbon isotope composition were dried at 60°C. Approximately 2 to 2.5 mg of dried leaf material was placed in tin capsules and combusted in a hydrogen/carbon/nitrogen elemental analyzer (ECS 4010; Costech Analytical) to determine stable carbon isotope ratio. Aboveground biomass was harvested and weighed to determine fresh weight, then dried for several weeks at 60°C to determine dry weight.
Enzyme Assays
Leaf samples for enzyme activity assays were collected as described above for stomatal density. Enzyme assays were performed at 25°C and pH 8 as described previously (Studer et al., 2016).
Drought Experiment
Two hundred Z. mays kernels segregating 1:2:1 (wild type:heterozygous:homozygous ca1ca2) from a single ear were planted in deep-well plug trays in MetroMix 360 with Turface (3:1 ratio). Seedlings were germinated in the greenhouse with 14-h days at 28°C and 10-h nights at 22°C with 50% relative humidity. Natural light was supplemented with a mixture of high-pressure sodium and metal halide fixtures. DNA for genotyping was extracted using a CTAB method on a 2-cm2 piece of tissue from the first leaf of 10-d-old seedlings. Wild-type and homozygous ca1ca2 mutant plants were transplanted after 2 weeks into 2.5-gallon black plastic pots. Before transplanting, pots were filled with Fafard 51 Custom Mix and dried at 60°C for 1 week. The pots were then removed from the dryer and immediately normalized to 2 kg. This was done so that a uniform amount of soil was added to each pot. Pots were then soaked in deionized water until saturated to determine the FC. Plants were transplanted and watered to saturation. A granulated starter fertilizer, consisting of one teaspoon of granulated fertilizer (600 mL [one part] Osmocote [15-9-12], 600 mL [one part] Tomato Maker [4-2-6 ca mg], 50 mL [one-twelfth part] Sprint, and one tablespoon of ferrous sulfate) was added as a top dressing to each pot after transplanting. Pots containing seedlings were then moved to a greenhouse with 14-h days at 32°C and 10-h nights at 22°C with 30% relative humidity for the duration of the experiment. Natural light was supplemented with metal halide fixtures at an intensity of 590 μmol m−2 (light measurement taken at the pot level). For the first 3 weeks of the experiment, all pots were well watered with tempered water supplemented with 200 µL L−1 15:5:15 (N:P:K) cal-mag fertilizer. Eight to 10 replicates were grown per genotype in each treatment. Five representative pots were weighed at saturation to determine the well-watered (FC) weight as 4.5 kg or gravimetric water content of 1.25 g water g−1 soil. Three weeks after planting, each pot was watered to FC; then, the pots were allowed to dry down to begin the drought treatment. Pots were weighed daily, and water was added on an individual pot basis. Daily transpiration was measured as the difference in pot weight between measurements, taking into account soil evaporation (differences in pot weight without a plant).
Accession Numbers
Sequence data referred to in this article can be found in the Gramene or Phytozome databases under accession numbers GRMZM2G121878 (Ca1) and GRMZM2G348512 (Ca2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Water concentration and leaf-to-air VPD during stomatal response measurements.
Supplemental Figure S2. Steady-state gas-exchange measurements during the CO2 response.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Lwanga Nsubuga for assistance with gas-exchange measurements and Jiayang Xie and Andrew Leakey for assisting with stomatal density measurements.
Footnotes
This work was supported by the National Science Foundation (grant nos. IOS-1127017 and IOS-1314143 to T.P.B. and Major Research Instrumentation grant no. DBI0923562 to A.B.C.) and by the U.S. Department of Energy (Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, Photosynthetic Systems, grant no. DE-SC0001685 to A.B.C., and Division of Biosciences, Life Sciences Research Foundation Postdoctoral Fellowship, grant no. DE-FG02-12ER16337 to A.J.S.).
References
- Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Badger MR. (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45: 369–392 [Google Scholar]
- Chen T, Wu H, Wu J, Fan X, Li X, Lin Y (2017) Absence of OsβCA1 causes a CO2 deficit and affects leaf photosynthesis and the stomatal response to CO2 in rice. Plant J 90: 344–357 [DOI] [PubMed] [Google Scholar]
- DiMario RJ, Quebedeaux JC, Longstreth DJ, Dassanayake M, Hartman MM, Moroney JV (2016) The cytoplasmic carbonic anhydrases βCA2 and βCA4 are required for optimal plant growth at low CO2. Plant Physiol 171: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK (1993) Evolutionary and ecological aspects of photosynthetic pathway variation. Annu Rev Ecol Syst 24: 411–439 [Google Scholar]
- Engineer CB, Ghassemian M, Anderson JC, Peck SC, Hu H, Schroeder JI (2014) Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 513: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre N, Reiter IM, Becuwe-Linka N, Genty B, Rumeau D (2007) Characterization and expression analysis of genes encoding α and β carbonic anhydrases in Arabidopsis. Plant Cell Environ 30: 617–629 [DOI] [PubMed] [Google Scholar]
- Gillon JS, Yakir D (2000) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ 23: 903–915 [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8: 391–397 [DOI] [PubMed] [Google Scholar]
- Hoang CV, Chapman KD (2002) Biochemical and molecular inhibition of plastidial carbonic anhydrase reduces the incorporation of acetate into lipids in cotton embryos and tobacco cell suspensions and leaves. Plant Physiol 128: 1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Rappel WJ, Occhipinti R, Ries A, Böhmer M, You L, Xiao C, Engineer CB, Boron WF, Schroeder JI (2015) Distinct cellular locations of carbonic anhydrases mediate carbon dioxide control of stomatal movements. Plant Physiol 169: 1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203: 1064–1081 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Scholes JD, Press MC (2005) Physiological and ecological significance of sunflecks for dipterocarp seedlings. J Exp Bot 56: 469–482 [DOI] [PubMed] [Google Scholar]
- Li P, Ponnala L, Gandotra N, Wang L, Si Y, Tausta SL, Kebrom TH, Provart N, Patel R, Myers CR, et al. (2010) The developmental dynamics of the maize leaf transcriptome. Nat Genet 42: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL (2014) Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344: 516–519 [DOI] [PubMed] [Google Scholar]
- Osborn HL, Alonso-Cantabrana H, Sharwood RE, Covshoff S, Evans JR, Furbank RT, von Caemmerer S (2017) Effects of reduced carbonic anhydrase activity on CO2 assimilation rates in Setaria viridis: a transgenic analysis. J Exp Bot 68: 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle HH, Hedrich R (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Sage RF. (2004) The evolution of C4 photosynthesis. New Phytol 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Studer AJ, Gandin A, Kolbe AR, Wang L, Cousins AB, Brutnell TP (2014) A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant Physiol 165: 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer AJ, Schnable JC, Weissmann S, Kolbe AR, McKain MR, Shao Y, Cousins AB, Kellogg EA (2016) The draft genome of Dichanthelium oligosanthes: a C3 panicoid grass species. Genome Biol 17: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S. (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia [Google Scholar]
- von Caemmerer S, Quinn V, Hancock NC, Price GD, Furbank RT, Ludwig M (2004) Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant Cell Environ 27: 697–703 [Google Scholar]
- Wang L, Czedik-Eysenberg A, Mertz RA, Si Y, Tohge T, Nunes-Nesi A, Arrivault S, Dedow LK, Bryant DW, Zhou W, et al. (2014) Comparative analyses of C4 and C3 photosynthesis in developing leaves of maize and rice. Nat Biotechnol 32: 1158–1165 [DOI] [PubMed] [Google Scholar]
- Zhang N, Gibon Y, Wallace JG, Lepak N, Li P, Dedow L, Chen C, So YS, Kremling K, Bradbury PJ, et al. (2015) Genome-wide association of carbon and nitrogen metabolism in the maize nested association mapping population. Plant Physiol 168: 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]