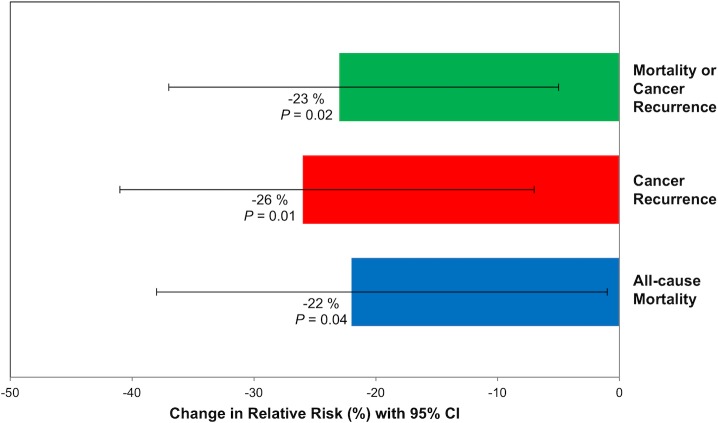
Fig 3. Results of isovolumetric substitution analysis, calculating change in relative risk of patient outcomes with substitution of 1 serving/day of artificially sweetened beverage for sugar-sweetened beverage.
Change in relative risk is displayed for each outcome as a percentage both graphically and in text, with 95% confidence intervals depicted graphically as error bars. Adjusted for age (continuous variable in years), sex (male or female), depth of invasion through bowel wall (binary variable, pT1-2 or pT3-4), number of positive lymph nodes (binary variable, 1–3 nodes or ≥4 nodes), baseline performance status (binary variable, 0 or 1–2), chemotherapy treatment group (binary variable, 5-fluorouracil and leucovorin or irinotecan, 5-fluorouracil, and leucovorin), consistent aspirin use (yes on both questionnaire 1 and 2), and time-varying total calorie intake (continuous variable, in kilocalories per day), physical activity (continuous variable in metabolic equivalent task hours per week), and body mass index (continuous variable in kilogram per meter-squared). P values are two-sided. Abbreviations: CI, confidence interval.