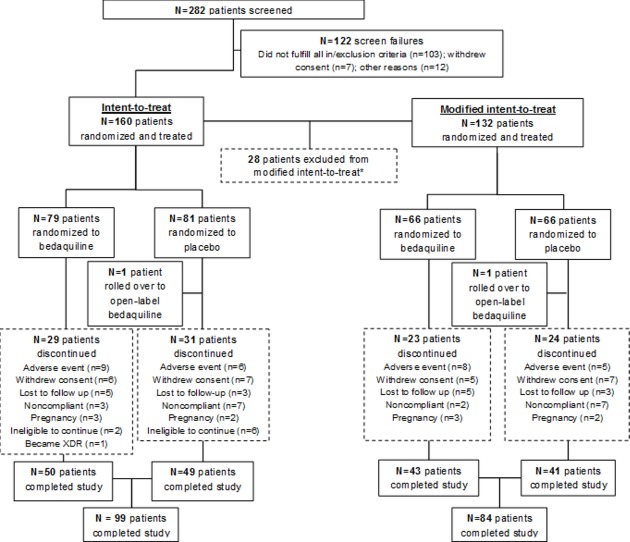
Fig 1. CONSORT flow diagram for the C208 Stage II trial [19].
*The modified intent-to-treat population was a subset of the intent-to-treat population that excluded nine patients (6 BDQ and 3 placebo) with Mycobacteria Growth Indicator Tube results that did not allow for primary efficacy evaluation (no evidence of culture positivity prior to first intake of blinded study drug or no results during the first 8 weeks after first intake), seven patients (3 BDQ and 4 placebo) infected with extensively drug-resistant tuberculosis, eight (4 BDQ and 4 placebo) with drug-sensitive tuberculosis, and four patients (0 BDQ and 4 placebo) for whom the multidrug-resistant tuberculosis status could not be confirmed.