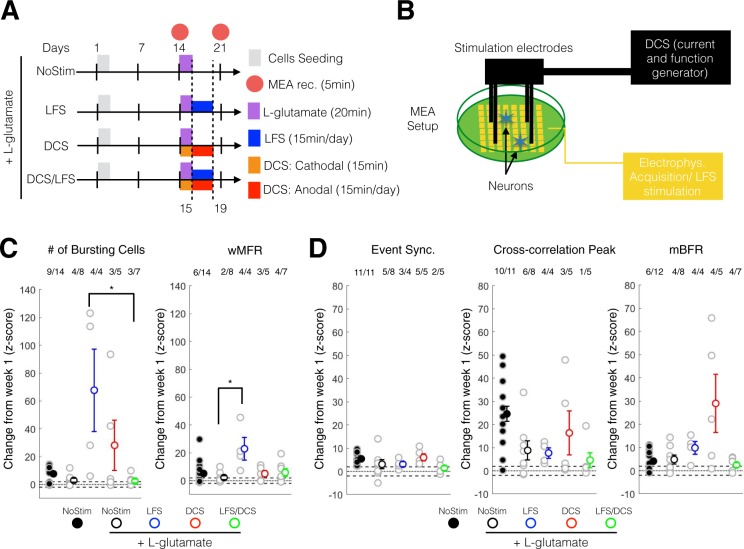
Figure 6.
DCS and LFS stimulation enhanced the maturation in excitability and neural synchrony following acute L-glutamate treatment. (A) Experimental schedule for ESC-derived neuron seeding, culture and treatment. L-glutamate (100 µM) was administered at day14 for 20 min then washed out. LFS was administered from day 15 to day 19 using 10 µA at 0.1 Hz, 15 min/day. DCS was administered in two phases: day14 a cathodal stimulation was performed for 15 min; from day 15 to day 19, anodal stimulation was administered for 15 min per day. Recording was performed on day 14 (week 2) and day 21 (week 3). (B) Schematic set up for electrical stimulation on MEA plate. LFS stimulation (10 µA, 0.1 Hz, 15 min/day) was delivered through the MEA electrode using the Maestro system. Four stainless screws were positioned above the MEA cultured neurons and were used to deliver a controlled current (DCS: single-time 10 µA monophonic cathodal 15 min and daily 10 µA monophonic anodal current, 15 min/day) using a custom battery-powered system. (C) Change at week 3 (expressed as a Z-score from week1 baseline) of the number of bursting cells (left panel) and wMFR (right panel). For post-hoc multiple comparison using Dunn-Sidak correction *, ** and *** indicate p < 0.05, p < 0.01 and p < 0.001, respectively. wMFR: weighted mean population firing rate. (D) Change at week 3 (expressed as a Z-score from week1 baseline) of the event synchronization (left panel), cross-correlation peak (middle panel) and mBFR (right panel). For post-hoc multiple comparison using Dunn-Sidak correction *, ** and *** indicate p < 0.05, p < 0.01 and p < 0.001, respectively. mBFR: mean population burst firing rate.