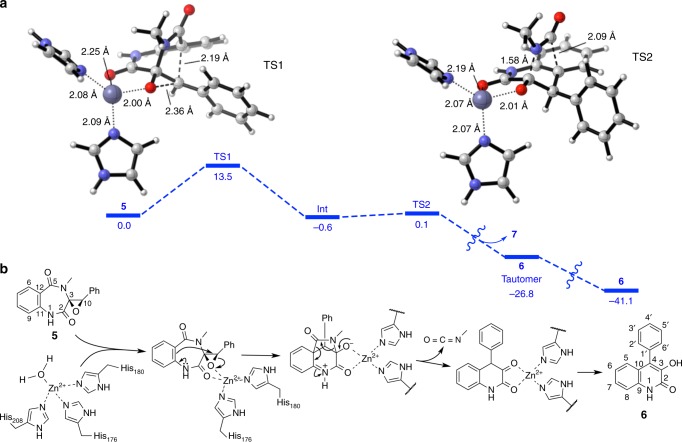
Fig. 4.
Computational analyses of the AsqI-catalyzed ring contraction reaction. a The metal-binding site of AsqI. Computational results on the full reaction pathway for the transformation of 5 into 6, catalyzed by a Zn2+ ion tetracoordinated to the substrate and two molecules of imidazole as a model for a histidine side chain. Calculated free energy differences (ΔG) in kcal mol–1 are given below the bold bars. The largest barrier is the first transition state (TS1) involving the opening of the cyclopenin epoxide and formation of the methylamide-bridged intermediate (Int). The second transition state (TS2) leading to the elimination of 7 and formation of the 6,6-bicyclic core is virtually barrierless. Computed TS1 and TS2 structures are shown. Carbon, oxygen, nitrogen and zinc atoms in the ball-and-stick models are in white, red, darker blue, and lighter blue, respectively. Breaking and forming bonds are shown as thick broken lines, while bonds between the zinc atom and other bound atoms are shown as thin dotted lines. b The proposed mechanism for the conversion of 5 to 6 catalyzed by the AsqI–Zn2+ complex. The Zn2+ ion bound to His176 and His180 is thought to catalyze the reaction as a Lewis acid