Abstract
Amides are ubiquitous in the fine chemical, agrochemical and pharmaceutical industries, but are rarely exploited as substrates for homologous amine synthesis. By virtue of their high chemical stability, they are essentially inert to all but the harshest of chemical reagents and to the majority of chemical transformations routinely used in organic synthesis. Accordingly, the development of chemoselective carbon−carbon bond-forming methodologies arising from the functionalization of the amide functionality should find widespread use across academia and industry. We herein present our findings on a series of Ugi-type reactions of tertiary amides enabled by an initial chemoselective iridium-catalyzed partial reduction, followed by reaction with isocyanide and (thio)acetic acid or trimethylsilyl azide, thus providing a multicomponent synthesis of α-amino (thio)amide or α-amino tetrazole derivatives. The reductive Ugi-type reactions are amenable to a broad range of amides and isocyanides, and are applicable to late-stage functionalization of various bioactive molecules and pharmaceutical compounds.
Chemical transformation of amides is normally occurring under harsh conditions. Here, the authors report a mild iridium-catalyzed reductive Ugi-type coupling of tertiary amides, isocyanides and (thio)acetic acid or trimethylsilyl azide to give homologous, bioactive amine products.
Introduction
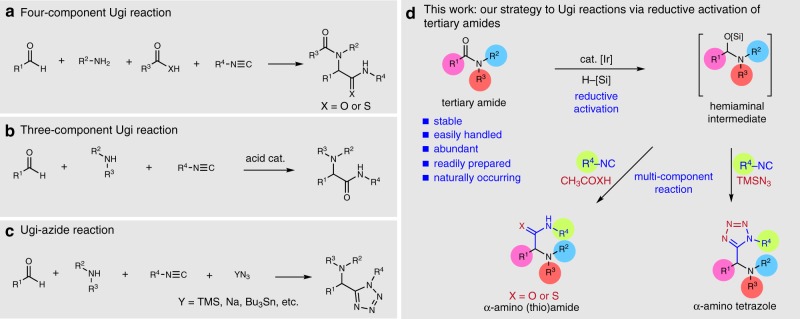
Multicomponent reactions (MCRs) have long been among the most attractive reactions in organic and medicinal chemistry, given their power of rapidly generating structural and chemical diversity1,2. Ugi reactions (Fig. 1a–c), which command a privileged place in the development of modern MCRs3–6, classically include three-component (3-MCRs) and four-component (4-MCRs) types according to the number of distinct reactant classes involved7. These reactions require reactive aldehydes and amines (mainly primary)8,9 to form the key imine/iminium intermediates, which are subsequently trapped by isocyanide nucleophiles, and afford either α-amino thioamides or α-amino amides depending on the nature of the carboxylic (thio)acid used to promote the reaction10,11. In a related variant, the combination of aldehyde, amine, and trimethylsilyl azide (TMSN3) can produce efficiently α-amino tetrazole derivatives. This MCR—generally referred to as the Ugi-azide reaction—has become established as one of the most reliable and versatile strategies to access such compounds12. Tetrazoles are an important class of compound commonly used within drug discovery programs13,14, as bioisosteres for the carboxylate group and, for example, as blockers for the Angiotensin II receptor15. Furthermore, various tetrazole-containing molecules have found use in organocatalysis16 and organometallic chemistry17, as well as in material science18.
Fig. 1.
Proposed reductive Ugi-type reactions of tertiary amides. a Classical four-, b three-component Ugi reactions, and c Ugi-azide reaction, d our proposed reductive Ugi-type reactions of tertiary amides
Tertiary amides are an under-exploited class of nitrogen-containing compound that is widespread throughout the chemical, pharmaceutical and agrochemical industries. They can be readily prepared from commonly occurring and abundant carboxylic acids and amines through well-defined, reliable coupling methods19,20, and—due to their high chemical stability and low inherent reactivity—they are essentially inert to all but the harshest of reaction types. Accordingly, new strategies for the chemoselective functionalization of amides, leading to new carbon−carbon bond-forming methodologies, would find tremendous application in synthesis, late-stage functionalization, and rapid generation of molecular diversity21–42.
Our group43–45 has been working on developing new methodologies for the synthesis of amine-containing products from reactive hemiaminal intermediates, generated by the highly chemoselective, catalytic reduction of tertiary amides under mild conditions46–50. Looking to expand the diversity of product types accessible we considered isocyanides51–53 as potential nucleophiles. Based on the known interaction between hemiaminals10 and isocyanides and our own prior experience with both partners43–45,54–56, we hypothesized that isocyanides might, in the presence of an acid promoter, intercept the in situ formed iminium species, thus generating versatile nitrilium intermediates applicable for various Ugi-type reactions (Fig. 1d). In related context, Zheng and Huang have reported that secondary amides undergo Ugi-amide formation when activated with triflic anhydride30.
Herein we report our findings leading to an iridium-catalyzed reductive Ugi-type MCR of tertiary amides, isocyanide and (thio)acetic acid or trimethylsilyl azide (TMSN3), affording α-amino (thio)amide and α-amino tetrazole structures respectively.
Results
Optimization study
As a proof-of-concept study, 4-methylbenzoxyl amide (1.0 eq) was subjected to standard amide partial reduction condition using IrCl(CO)(PPh3)2 (Vaska’s complex, 1 mol%) and tetramethyldisiloxane (TMDS, 2.0 eq) in dichloromethane at room temperature for 20 min, followed by the addition of tert-butyl isocyanide (2.0 eq) and formic acid (1.0 eq). We were pleased to detect the desired α-amino amide product 2 in significant amounts (Table 1, entry 1). The reaction was also successful using acetic acid (1.0 eq) in place of formic acid; 1H NMR analysis indicated a 75% yield of the product (Table 1, entry 2). Further experiments demonstrated a slight excess of acetic acid resulted in the formation of 2 in 78% isolated yield (Table 1, entries 3−5). Reaction concentration (Table 1, entries 6, 7), solvents (Table 1, entries 8, 9) and acids (Table 1, entries 10−13) were also screened, but the optimal conditions were those as described in entry 4.
Table 1.
Discovery and optimization
|
| |||
|---|---|---|---|
| Entry | Solvent (M) | Acid (eq) | Yield (%)a |
| 1 | CH2Cl2 (0.1) | HCOOH (1.0) | 35 |
| 2 | CH2Cl2 (0.1) | CH3COOH (1.0) | 75 |
| 3 | CH2Cl2 (0.1) | CH3COOH (0.2) | 31 |
| 4 | CH2Cl2 (0.1) | CH3COOH (1.2) | 82 (78b) |
| 5 | CH2Cl2 (0.1) | CH3COOH (2.0) | 78 |
| 6 | CH2Cl2 (0.2) | CH3COOH (1.2) | 80 |
| 7 | CH2Cl2 (0.05) | CH3COOH (1.2) | 20 |
| 8 | DCE (0.1) | CH3COOH (1.2) | 76 |
| 9 | toluene (0.1) | CH3COOH (1.2) | 78 |
| 10 | CH2Cl2 (0.1) | diphenyl phosphate (1.2) | <10 |
| 11 | CH2Cl2 (0.1) | camphorsulphonic acid (1.2) | trace |
| 12 | CH2Cl2 (0.1) | 4-nitrophenol (1.2) | N.O. |
| 13 | CH2Cl2 (0.1) | ZnCl2 (1.2) | N.O. |
Reactions were performed on 0.3 mmol of amide
N.O. not observed
aNMR yields with 1-bromo-2-methoxylnaphthlene as internal standard
bIsolated yield. DCE: 1,2-dichloroethane
Substrate scope
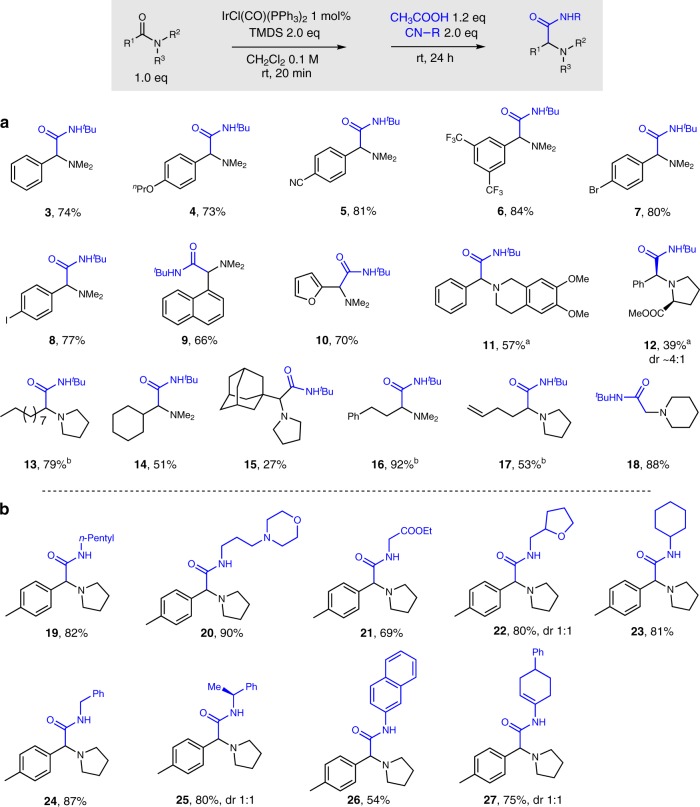
With optimal conditions in hand, we then explored the scope of this reductive Ugi reaction by subjecting a broad range of carboxylic amides to this protocol. As shown in Fig. 2a, aryl amides with both electron-donating and electron-withdrawing groups on the phenyl ring were good substrates affording the corresponding amino amides (3−8) in good yields. The toleration of aryl bromide and aryl iodide residues indicates the possibility of combining this protocol with standard metal-catalyzed cross-coupling chemistry. Naphthalene and furan-derived amides underwent the transformation smoothly (9, 10). Amides derived from dimethoxy-1,2,3,4-tetrahydroisoquinoline and proline also proved amenable and allowed the synthesis of amino amide (11, 12) albeit using a slightly increased temperature (50 °C). Linear alkyl, α-branched and fully substituted alkyl amides were all viable partners, delivering the corresponding products (13−15). Diminished yields were observed when the substrates were sterically hindered. Pendant phenyl and vinyl groups in the carboxylic acid residue were also tolerated and delivered products (16, 17). Moreover, the use of a formic acid-derived amide led to the unsubstituted α-amino amide in excellent yield (18). Unfortunately, attempts to apply lactam substrates, such as 1-butylazepan-2-one and 1-benzylpiperidin-2-one, to this reductive Ugi-amide formation were unsuccessful.
Fig. 2.
Synthesis of α-amino amides. a Scope with respect to the tertiary amide. b Scope with respect to the isocyanide. Standard condition: amide 0.3 mmol, IrCl(CO)(PPh3)2 1 mol%, TMDS 0.6 mmol, CH2Cl2 3 mL, isocyanide 0.6 mmol, CH3COOH 0.36 mmol; yields of purified product following flash column chromatography; areaction was performed at 50 °C after the addition of tert-butyl isocyanide and CH3COOH; bwith 0.9 mmol CH3COOH
Subsequently, the scope of the reaction with respect to the isocyanide component was examined under the standard reaction conditions (Fig. 2b). Linear alkyl isocyanides bearing morpholino, ester, and tetrahydrofuranyl functionality were excellent partners allowing the ready assembly of amino amides (19−22). Branched cyclohexyl and benzyl isocyanide afforded good yields of the respective MCR products (23, 24). Good reactivity, albeit with no diastereoselectivity, was found when enantiopure α-methylbenzyl isocyanide was applied (25). Furthermore, both aryl and vinyl amides were obtained in good yields (26, 27) when the corresponding isocyanides were submitted to the standard reaction conditions.
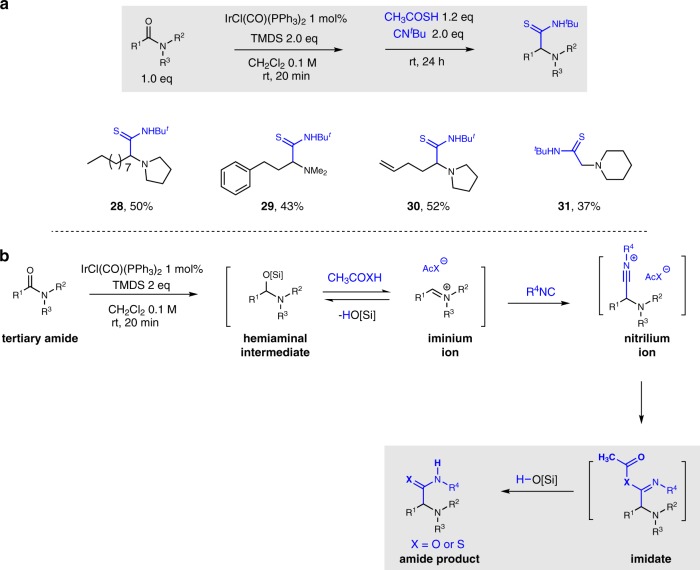
As is classically known for Ugi-type reactions, use of thioacetic acid as a substitute for acetic acid should result in the generation of thioamide functionality in the reaction product8. To our delight, the corresponding thioamides were indeed isolated with reasonable yields when a range of tertiary amide substrates were submitted to the standard condition, but with 1.2 eq CH3COSH as the acid to promote the Ugi reaction (28−31) (Fig. 3a). These results indicate further synthetic applications of this reductive Ugi protocol.
Fig. 3.
Synthesis of α-amino thioamides and mechanism of the reductive Ugi reactions. a Synthesis of α-amino thioamide derivatives from tertiary amides. Standard condition: amide 0.3 mmol, IrCl(CO)(PPh3)2 1 mol%, TMDS 0.6 mmol, CH2Cl2 3 mL, tert-butyl isocyanide 0.6 mmol, CH3COSH 0.36 mmol; yields of purified product following flash column chromatography. b Proposed mechanism of the reductive Ugi reactions
Regarding the reaction mechanism for the formation of α-amino (thio)amides and the role of (thio)acetic acid throughout the transformation, a plausible reaction pathway is proposed in Fig. 3b. The silylated hemiaminal intermediate is initially generated by the standard reductive condition from the amide starting material. Then by adding the isocyanide and (thio)acetic acid, the hemiaminal is transformed into the highly reactive nitrilium intermediate10, either directly or via the corresponding iminium ion. Nucleophilic addition by acetate or thioacetate then affords the (thio)imidate intermediate, which after rearrangement, leads to the observed α-amino amide or thioamide product8.
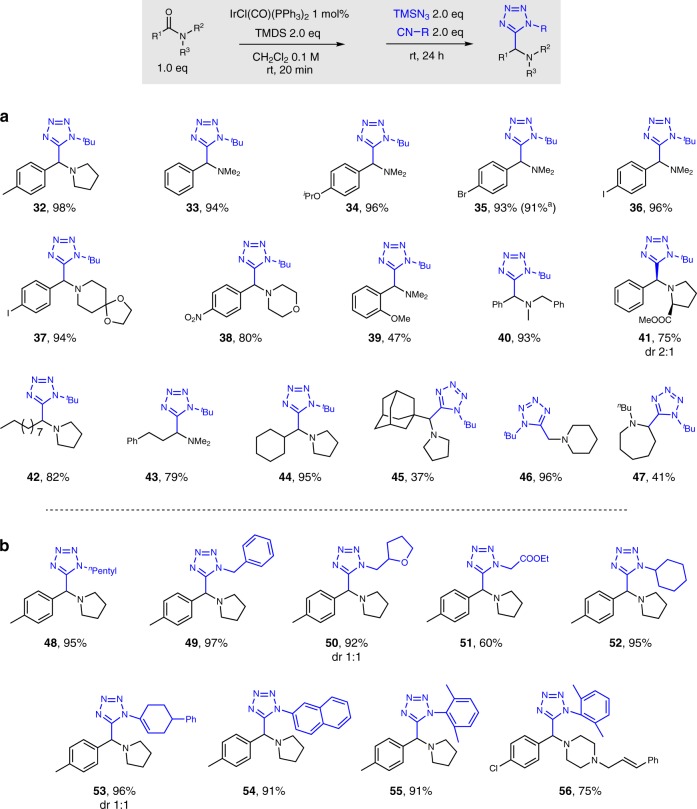
These exciting successes inspired us to hypothesize that the formed reactive nitrilium intermediate might also be poised to undergo the Ugi-azide reaction: trapping by an appropriate azide source should facilitate a 1,3-dipolar cyclization, thus delivering α-amino tetrazole as the product57–60. This reaction would transform stable, readily available tertiary amides into homologated high value α-amino tetrazole motifs in a single pot. The idea was initially tested by mixing tert-butyl isocyanide, acetic acid, and TMSN3 with the in situ generated hemiaminal from amide 1 under the standard and mild reaction conditions. Indeed the Ugi-azide reaction proceeded smoothly, leading to the α-amino tetrazole product 32 in 90% yield. Further follow-up experiments demonstrated that without the addition of acetic acid, the tetrazole product was obtained in quantitative yield (98%).
Figure 4a depicts the full scope of tertiary amides compatible with this α-amino tetrazole synthesis. Both electron-rich and electron-deficient phenyl derived amides smoothly coupled with azide and isocyanide under this protocol, delivering excellent yields of the desired products (32−38). Steric hindrance, at the ortho-position of benzoyl amide derivatives, led to a decline in yield (39). N-Benzyl amine-derived amide proved to be a suitable partner for the amide Ugi-azide reaction (40). It is worth noting that N-benzoyl methylprolinate was also a good substrate, giving proline-derived tetrazole product in 75% yield (dr 2:1) (41). The tetrazole synthesis also tolerated linear and α-branched alkyl amides (42−44). Tertiary alkyl amide, as exemplified by adamantanyl dimethyl amide, was also amenable to the protocol (45), although a lower yield of product was obtained. An excellent yield of α-amino tetrazole product was obtained when a formamide substrate was employed (46). Importantly, we found a lactam substrate was also applicable to this reaction, although only a moderate yield of product 47 was obtained. In addition, 91% of the tetrazole product 35 was isolated upon exposing 1.14 g of the corresponding amide to the standard reaction condition, proving that the reaction can be readily carried out on gram scale.
Fig. 4.
Synthesis of α-amino tetrazoles. a Scope with respect to the tertiary amide. b Scope with respect to the isocyanide. Standard condition: amide 0.3 mmol, IrCl(CO)(PPh3)2 1 mol%, TMDS 0.6 mmol, CH2Cl2 3 mL, isocyanide 0.6 mmol, trimethylsilyl azide 0.6 mmol; yields of purified product following flash column chromatography; areaction was carried out with 1.14 g of amide
Further studies focused on the scope with respect to the isocyanide component of this reductive Ugi-azide reaction of tertiary amides (Fig. 4b). Primary isocyanides bearing functional groups such as phenyl, tetrahydrofuran, and ester functionality were well-tolerated under the standard conditions (48−51). Cyclohexyl isocyanide (an example of secondary alkyl system) delivered the tetrazole product with 95% yield (52). Olefinic and aryl isocyanides afforded the corresponding products in excellent yields (53−55); even sterically demanding systems such as the 2,6-dimethylphenyl isocyanide had no deleterious effect on the efficiency of this reaction (55). This amide reductive Ugi-azide reaction will likely find various applications for the direct synthesis of pharmaceutical agents, and to this end here we have demonstrated the one-pot preparation of tetrazole compound 56, an MCH1 receptor antagonist61, in 75% yield starting from a stable, readily available amide.
Synthetic utility
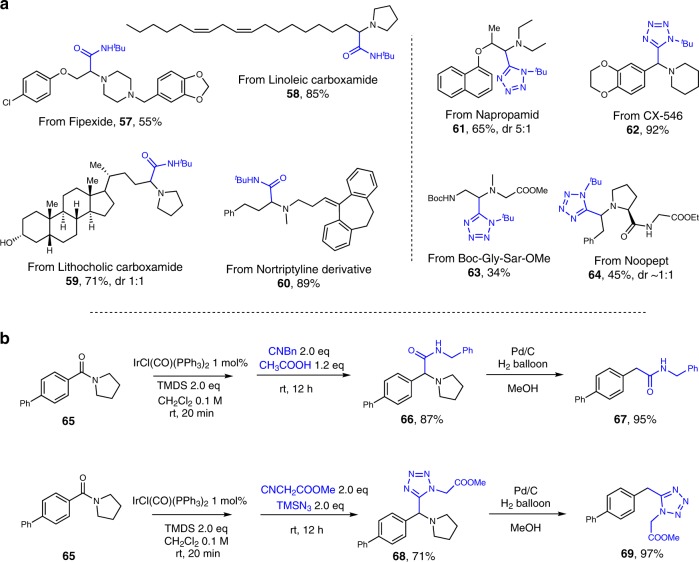
Given the prevalence of tertiary amides and their precursors in bioactive molecules, and the surprisingly broad scope tolerated in these amide reductive Ugi-type (thio)amide and tetrazole syntheses, further synthetic potential of this chemistry is demonstrated by the late-stage functionalization of various bioactive molecules and derivatives (Fig. 5a). Fipexide62, a psychoactive drug, could be readily coupled with tert-butyl isocyanide in the presence of acetic acid (57). Linoleic carboxamide, readily prepared from one of the essential fatty acids, linoleic acid63, underwent the α-amino amide formation in 85% yield (58). Lithocholic acid64 (LCA), known for its ability to selectively kill neuroblastoma cells, could be transformed into the corresponding Ugi amide product in a similar fashion (59). While the carboxamide derivative of the amine drug nortriptyline65, used for treatment of clinical depression, was successfully modified, delivering the desired product in 89% yield (60). Submission of the selective systemic amide herbicide, napropamid66, to the amide reductive Ugi-azide reaction resulted in 65% yield of its tetrazole derivative 61, with 5:1 ratio of diastereoisomers. The reductive coupling of CX-546, a drug candidate for schizophrenia67, with tert-butyl isocyanide and TMSN3 led to the corresponding product in excellent yield (62). The success of dipeptide Boc-Gly-Sar-OMe modification under these conditions (63) encouraged us to apply this procedure to the peptide drug noopept (omberacetam, a nootropic)68. Thus, we were pleased to obtain the tetrazole derivative of noopept in 45% yield (64).
Fig. 5.
Synthetic application. a Late-stage functionalization of bioactive molecules and derivatives using amide reductive Ugi-type reactions. b Synthesis of bioactive molecules via reductive Ugi-type reactions followed by hydrogenation. Standard condition: amide 0.3 mmol, IrCl(CO)(PPh3)2 1 mol%, TMDS 0.6 mmol, CH2Cl2 3 mL, isocyanide 0.6 mmol, CH3COOH 0.36 mmol or trimethylsilyl azide 0.6 mmol; yields of purified product following flash column chromatography
Furthermore, by submitting amide 65 (Fig. 5b) to the standard procedure of α-amino amide synthesis, the corresponding amide product was obtained. Removal of pyrrolidine from compound 66 took place nearly quantitatively in the presence of palladium on carbon under an atmosphere of hydrogen, enabling the synthesis of 67 (patented name: IND116065), which exhibits activity in cell viability inhibition69. Analogously, regioselective synthesis of 1,5-disubstituted tetrazole compound 69, previously made as an enzyme inhibitor70, could be achieved by carrying out our reductive Ugi-azide reaction, in the presence of corresponding isocyanide and TMSN3, followed by the hydrogenation.
In summary, a series of reductive Ugi-type reactions, leading to homologated α-amino (thio)amide and tetrazole motifs, by merging hemiaminal intermediates, isocyanides and (thio)acetic acid or TMSN3, has been developed. These reactions benefit from the strategic use of stable and readily available tertiary amides as abundant and under-exploited nitrogen-containing starting materials. The broad scope of tertiary amides and isocyanides that can be tolerated demonstrate the practicality of these multicomponent reactions. Combining this with the illustrated synthetic utility by late-stage functionalization of various bioactive molecules and derivatives, as well as the synthesis of bioactive compounds possessing amide and tetrazole functionality, this powerful α-amino amide and α-amino tetrazole synthesis strategy will likely find numerous applications across academia and industry.
Methods
General procedure for the synthesis of α-amino amides and tetrazoles from tertiary amides
Vaska’s catalyst (2.4 mg, 1 mol%) and amide (0.3 mmol, 1.0 eq) were charged into a dry 25 mL flask. Vacuum and N2 refilling were repeated for three times. Dry CH2Cl2 (3 mL, 0.1 M) was injected by syringe, and then TMDS (0.6 mmol, 2.0 eq), while stirring was maintained at room temperature. The resulting mixture was stirred for 20 min, then isocyanide (0.6 mmol, 2.0 eq) and acetic acid (0.36 mmol, 1.2 eq) or TMSN3 (0.6 mmol, 2.0 eq) were added sequentially. The solution was stirred overnight at room temperature and was then quenched with saturated aqueous NaHCO3 solution and extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over Na2SO4 and filtered. The solvent was removed under vacuum. The residue was purified by flash column chromatography.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and the Supplementary Information, as well as from the authors upon reasonable request.
Electronic supplementary material
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 707559-H2020-MSCA-IF-2015 (to L.-G.X.), and the EPSRC (Leadership Fellowship to D.J.D.).
Author contributions
L.-G.X. and D.J.D. planned the project. L.-G.X. carried out the experiments and analyzed the data. L.-G.X. and D.J.D. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05192-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu J, Wang Q, Wang MX. Multicomponent Reactions in Organic Synthesis. Weinheim: Wiley-VCH; 2015. [Google Scholar]
- 2.Zhu J, Bienayme H. Multicomponent Reactions. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 3.Dömling A, Wang W, Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruijter E, Scheffelaar R, Orru RVA. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 2011;50:6234–6246. doi: 10.1002/anie.201006515. [DOI] [PubMed] [Google Scholar]
- 5.Wessjohann LA, Rivera DG, Vercillo OE. Multiple multicomponent macrocyclizations (MiBs): a strategic development toward macrocycle diversity. Chem. Rev. 2009;109:796–814. doi: 10.1021/cr8003407. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen TE, Schreiber SL. Towards the optimal screening collection: a synthesis strategy. Angew. Chem. Int. Ed. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milen M, Dancso A, Foldesi T, Volk B. Study on the propylphosphonic anhydride (T3P®) mediated Ugi-type three-component reaction. Efficient synthesis of an α-amino amide library. Tetrahedron. 2017;73:70–77. doi: 10.1016/j.tet.2016.11.059. [DOI] [Google Scholar]
- 8.Kaim LE, Grimaud L. Beyond the Ugi reaction: less conventional interactions between isocyanides and iminium species. Tetrahedron. 2009;65:2153–2171. doi: 10.1016/j.tet.2008.12.002. [DOI] [Google Scholar]
- 9.Tanaka Y, Hasui T, Suginome M. Acid-free, aminoborane-mediated Ugi-type reaction leading to general utilization of secondary amines. Org. Lett. 2007;9:4407–4410. doi: 10.1021/ol701570c. [DOI] [PubMed] [Google Scholar]
- 10.Cheron N, Ramozzi R, El Kaim L, Grimaud L, Fleurat-Lessard P. Challenging 50 years of established views on Ugi reaction: a theoretical approach. J. Org. Chem. 2012;77:1361–1366. doi: 10.1021/jo2021554. [DOI] [PubMed] [Google Scholar]
- 11.Ugi I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. Eng. 1962;1:8–21. doi: 10.1002/anie.196200081. [DOI] [Google Scholar]
- 12.Maleki A, Sarvary A. Synthesis of tetrazoles via isocyanide-based reactions. RSC Adv. 2015;5:60938–60955. doi: 10.1039/C5RA11531K. [DOI] [Google Scholar]
- 13.Ostrovskii VA, Trifonov RE, Popova EA. Medicinal chemistry of tetrazoles. Russ. Chem. Bull. Int. Ed. 2012;61:768–780. doi: 10.1007/s11172-012-0108-4. [DOI] [Google Scholar]
- 14.Myznikov LV, Hrabalek A, Koldobskii GI. Drugs in the tetrazole series. Chem. Heterocycl. Compd. 2007;43:1–9. doi: 10.1007/s10593-007-0001-5. [DOI] [Google Scholar]
- 15.Julius S, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. New Engl. J. Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 16.Longbottom DA, Franckevičius V, Ley SV. (S)- and (R)-5-Pyrrolidin-2-yl-1H-tetrazoles: enanti-omeric organocatalysts of broad utility in organic synthesis. Chimia (Aarau) 2007;61:247–256. doi: 10.2533/chimia.2007.247. [DOI] [Google Scholar]
- 17.Aromí G, Barrios LA, Roubeau O, Gamez P. Triazoles and tetrazoles: prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011;255:485–546. doi: 10.1016/j.ccr.2010.10.038. [DOI] [Google Scholar]
- 18.Gao H, Shreeve JM. Azole-based energetic salts. Chem. Rev. 2011;111:7377–7436. doi: 10.1021/cr200039c. [DOI] [PubMed] [Google Scholar]
- 19.Pattabiraman VR, Bode JW. Rethinking amide bond synthesis. Nature. 2011;480:471–479. doi: 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]
- 20.Valeur E, Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009;38:606–631. doi: 10.1039/B701677H. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser D, Maulide N. Making the least reactive electrophile the first in class: domino electrophilic activation of amides. J. Org. Chem. 2016;81:4421–4428. doi: 10.1021/acs.joc.6b00675. [DOI] [PubMed] [Google Scholar]
- 22.Pace V, Holzer W, Olofsson B. Increasing the reactivity of amides towards organometallic reagents: an overview. Adv. Synth. Catal. 2014;356:3697–3736. doi: 10.1002/adsc.201400630. [DOI] [Google Scholar]
- 23.Volkov A, Tinnis F, Slagbrand T, Trilloa P, Adolfsson H. Chemoselective reduction of carboxamides. Chem. Soc. Rev. 2016;45:6685–6697. doi: 10.1039/C6CS00244G. [DOI] [PubMed] [Google Scholar]
- 24.Ruider SA, Maulide N. Strong bonds made weak: towards the general utility of amides as synthetic modules. Angew. Chem. Int. Ed. 2015;54:13856–13858. doi: 10.1002/anie.201508536. [DOI] [PubMed] [Google Scholar]
- 25.Addis D, Das S, Junge K, Beller M. Selective reduction of carboxylic acid derivatives by catalytic hydrosilylation. Angew. Chem. Int. Ed. 2011;50:6004–6011. doi: 10.1002/anie.201100145. [DOI] [PubMed] [Google Scholar]
- 26.Murai T, Asai F. Three-component coupling reactions of thioformamides with organolithium and Grignard reagents leading to formation of tertiary amines and a thiolating agent. J. Am. Chem. Soc. 2007;129:780–781. doi: 10.1021/ja068523f. [DOI] [PubMed] [Google Scholar]
- 27.Murai T, Mutoh Y, Ohta Y, Murakami M. Synthesis of tertiary propargylamines by sequential reactions of in situ generated thioiminium salts with organolithium and -magnesium reagents. J. Am. Chem. Soc. 2004;126:5968–5969. doi: 10.1021/ja048627v. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser D, Teskey CJ, Adler P, Maulide N. Chemoselective intermolecular cross-enolate-type coupling of amides. J. Am. Chem. Soc. 2017;139:16040–16043. doi: 10.1021/jacs.7b08813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tona V, et al. Chemo- and stere-oselective transition-metal-free amination of amides with azides. J. Am. Chem. Soc. 2016;138:8348–8351. doi: 10.1021/jacs.6b04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng JF, Qian XY, Huang PQ. Direct transformation of amides: a one-pot reductive Ugi-type three-component reaction of secondary amides. Org. Chem. Front. 2015;2:927–935. doi: 10.1039/C5QO00146C. [DOI] [Google Scholar]
- 31.Peng B, Fares C, Geerdink D, Maulide N. Chemoselective intermolecular α-arylation of amides. Angew. Chem. Int. Ed. 2014;53:5462–5466. doi: 10.1002/anie.201402229. [DOI] [PubMed] [Google Scholar]
- 32.Mewald M, Medley JW, Movassaghi M. Concise and enantioselective total synthesis of (−)-Mehranine, (−)-Methylenebismehranine, and related Aspidosperma alkaloids. Angew. Chem. Int. Ed. 2014;53:11634–11639. doi: 10.1002/anie.201405609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medley JW, Movassaghi M. A concise and versatile double-cyclization strategy for the highly ste-reoselective synthesis and arylative dimerization of Aspidosperma alkaloids. Angew. Chem. Int. Ed. 2012;51:4572–4576. doi: 10.1002/anie.201200387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao KJ, Wang AE, Huang PQ. Direct transformation of secondary amides into secondary amines: triflic anhydride activated reductive alkylation. Angew. Chem. Int. Ed. 2012;51:8314–8317. doi: 10.1002/anie.201204098. [DOI] [PubMed] [Google Scholar]
- 35.Bechara WS, Pelletier G, Charette AB. Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nat. Chem. 2012;4:228–234. doi: 10.1038/nchem.1268. [DOI] [PubMed] [Google Scholar]
- 36.Xiao KJ, Luo JM, Ye KY, Wang Y, Huang PQ. Direct, one-pot sequential reductive alkylation of lactams/amides with Grignard and organolithium reagents through lactam/amide activation. Angew. Chem. Int. Ed. 2010;49:3037–3040. doi: 10.1002/anie.201000652. [DOI] [PubMed] [Google Scholar]
- 37.Zheng JF, et al. Substrate-controlled chemoselective reactions of isocyanoacetates with amides and lactams. J. Org. Chem. 2017;82:9693–9703. doi: 10.1021/acs.joc.7b01768. [DOI] [PubMed] [Google Scholar]
- 38.Shirokane K, et al. Total syntheses of (±)-Gephyrotoxin and (±)-Perhydrogephyrotoxin. Bull. Chem. Soc. Jpn. 2015;88:522–537. doi: 10.1246/bcsj.20140398. [DOI] [Google Scholar]
- 39.Shirokane K, et al. Total synthesis of (±)-Gephyrotoxin by amide-selective reductive nucleophilic addition. Angew. Chem. Int. Ed. 2014;53:512–516. doi: 10.1002/anie.201308905. [DOI] [PubMed] [Google Scholar]
- 40.Jakubec P, Hawkins A, Felzmann W, Dixon DJ. Total synthesis of Manzamine A and related alkaloids. J. Am. Chem. Soc. 2012;134:17482–17485. doi: 10.1021/ja308826x. [DOI] [PubMed] [Google Scholar]
- 41.Vincent G, Guillot R, Kouklovsky C. Stereodivergent synthesis of substituted N,O-containing bicy-clic compounds by sequential addition of nucleophiles to N-alkoxybicyclolactams. Angew. Chem. Int. Ed. 2011;50:1350–1353. doi: 10.1002/anie.201006590. [DOI] [PubMed] [Google Scholar]
- 42.Shirokane K, Kurosaki Y, Sato T, Chida N. A direct entry to substituted N-methoxyamines from N-methoxyamides via N-oxyiminium ions. Angew. Chem. Int. Ed. 2010;49:6369–6372. doi: 10.1002/anie.201001127. [DOI] [PubMed] [Google Scholar]
- 43.Fuentes de Arriba ÁL, Lenci E, Sonawane M, Formery O, Dixon DJ. Iridium-catalyzed reductive Strecker reaction for late-stage amide and lactam cyanation. Angew. Chem. Int. Ed. 2017;56:3655–3659. doi: 10.1002/anie.201612367. [DOI] [PubMed] [Google Scholar]
- 44.Xie LG, Dixon DJ. Tertiary amine synthesis via reductive coupling of amides with Grignard reagents. Chem. Sci. 2017;8:7492–7497. doi: 10.1039/C7SC03613B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan PW, Seayad J, Dixon DJ. Expeditious and divergent total syntheses of Aspidosperma alkaloids exploiting iridium(I)-catalyzed generation of reactive enamine intermediates. Angew. Chem. Int. Ed. 2016;55:13436–13440. doi: 10.1002/anie.201605503. [DOI] [PubMed] [Google Scholar]
- 46.Motoyama Y, Aoki M, Takaoka N, Aoto R, Nagashima H. Highly efficient synthesis of aldenamines from carboxamides by iridium-catalyzed silane-reduction/dehydration under mild conditions. Chem. Commun. 2009;45:1574–1576. doi: 10.1039/b821317h. [DOI] [PubMed] [Google Scholar]
- 47.Yoritate M, et al. Unified total synthesis of Stemoamide-type alkaloids by chemoselective assembly of five-membered building blocks. J. Am. Chem. Soc. 2017;139:18386–18391. doi: 10.1021/jacs.7b10944. [DOI] [PubMed] [Google Scholar]
- 48.Katahara S, et al. An iridium-catalyzed reductive approach to nitrones from N-hydroxyamides. J. Am. Chem. Soc. 2016;138:5246–5249. doi: 10.1021/jacs.6b02324. [DOI] [PubMed] [Google Scholar]
- 49.Huang PQ, Ou W, Han F. Chemoselective reductive alkynylation of tertiary amides by Ir and Cu(I) bis-metal sequential catalysis. Chem. Commun. 2016;52:11967–11970. doi: 10.1039/C6CC05318A. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima M, Sato T, Chida N. Iridium-catalyzed chemoselective reductive nucleophilic addition to N-methoxyamides. Org. Lett. 2015;17:1696–1699. doi: 10.1021/acs.orglett.5b00664. [DOI] [PubMed] [Google Scholar]
- 51.Giustiniano M, et al. To each his own: isonitriles for all flavors. Functionalized isocyanides as valua-ble tools in organic synthesis. Chem. Soc. Rev. 2017;46:1295–1357. doi: 10.1039/C6CS00444J. [DOI] [PubMed] [Google Scholar]
- 52.Vlaar T, Ruijter E, Maes BUW, Orru RVA. Palladium-catalyzed migratory insertion of iso-cyanides: an emerging platform in cross-coupling chemistry. Angew. Chem. Int. Ed. 2013;52:7084–7097. doi: 10.1002/anie.201300942. [DOI] [PubMed] [Google Scholar]
- 53.Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 54.De la Campa R, et al. Catalytic enantio- and diastereoselective Mannich reaction of α-substituted iso-cyanoacetates and ketimines. Chem. Commun. 2016;52:10632–10635. doi: 10.1039/C6CC04132A. [DOI] [PubMed] [Google Scholar]
- 55.De La Campa R, Ortín I, Dixon DJ. Direct catalytic enantio- and diastereoselective ketone aldol reactions of isocyanoacetates. Angew. Chem. Int. Ed. 2015;54:4895–4898. doi: 10.1002/anie.201411852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortín I, Dixon DJ. Direct catalytic enantio- and diastereoselective Mannich reaction of isocyano-acetates and ketimines. Angew. Chem. Int. Ed. 2014;53:3462–3465. doi: 10.1002/anie.201309719. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Patil P, Kurpiewska K, Kalinowska-Tluscik J, Dömling A. Two cycles with one catch: hydrazine in Ugi 4-CR and its postcyclizations. ACS Comb. Sci. 2017;19:193–198. doi: 10.1021/acscombsci.7b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu G, Mamboury M, Wang Q, Zhu J. Ketenimines from isocyanides and allyl carbonates: palladium-catalyzed synthesis of β,γ-unsaturated amides and tetrazoles. Angew. Chem. Int. Ed. 2016;55:15377–15381. doi: 10.1002/anie.201609034. [DOI] [PubMed] [Google Scholar]
- 59.Kroon E, Kurpiewska K, Kalinowska-Tluscik J, Dömling A. Cleavable β-cyanoethyl isocyanide in the Ugi tetrazole reaction. Org. Lett. 2016;18:4762–4765. doi: 10.1021/acs.orglett.6b01826. [DOI] [PubMed] [Google Scholar]
- 60.Kalinski C, et al. A new and versatile Ugi/SNAr synthesis of fused 4,5-dihydrotetrazolo[1,5-a]quinoxalines. Tetrahedron Lett. 2006;47:2041–2044. doi: 10.1016/j.tetlet.2006.01.027. [DOI] [Google Scholar]
- 61.Hulme C., Tempest P., Ma V., Nixey T. & Balow G. Melanin Concentrating Hormone Receptor Antagonist. WO2005/019167 A2 (2005).
- 62.Missale C, Pasinetti G, Govoni S, Spano PF, Trabucchi M. Fipexide: a new drug for the regulation of dopaminergic system at the macromolecular level. Boll. Chim. Farm. 1983;122:79–85. [PubMed] [Google Scholar]
- 63.Burr GO, Burr MM, Miller E. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 1930;86:587–621. [Google Scholar]
- 64.Goldberg AA, et al. Lithocholic bile acid selectively kills neuroblastoma cells, while sparing normal neuronal cells. Oncotarget. 2011;2:761–782. doi: 10.18632/oncotarget.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brayfield, A. (ed.) Nortriptyline Hydrochloride—Martindale: The Complete Drug Reference. MedicinesComplete (Pharmaceutical Press, London, UK, 2017).
- 66.Thomas, A. Unger Pesticide Synthesis Handbook 41 (William Andrew, Norwich, NY, 1996).
- 67.Lipina T, Weiss K, Roder J. The Ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice. Neuropsychopharmacol. 2007;32:745–756. doi: 10.1038/sj.npp.1301191. [DOI] [PubMed] [Google Scholar]
- 68.Proposed INN List 117, Vol. 31, 308 (WHO Drug Information, Geneva, Switzerland, 2017).
- 69.Renslo, A. R. et al. Novel Antiprion Compounds. WO2013/033037 A2 (2013).
- 70.Ortar G, et al. New tetrazole-based selective anandamide uptake inhibitors. Bioorg. Med. Chem. Lett. 2008;18:2820–2824. doi: 10.1016/j.bmcl.2008.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and the Supplementary Information, as well as from the authors upon reasonable request.