Abstract
Background
Social isolation is associated with accelerated breast cancer progression and increased disease recurrence and mortality, but the underlying biological mechanisms remain poorly understood. In preclinical models, beta-adrenergic signaling from fight-or-flight stress responses can stimulate prometastatic processes in the tumor microenvironment including upregulation of M2 macrophages, epithelial–mesenchymal transition (EMT), and lymphovascular invasion. This study examines whether the same pathways are upregulated in breast tumors from socially isolated cancer patients.
Methods
EMT and M1/M2 macrophage gene expression programs were analyzed by genome-wide transcriptional profiling, and lymphatic and vascular density were assessed by immunohistochemistry in primary tumors from 56 early-stage breast cancer patients who were part of the UCLA RISE study. Social isolation was quantified by the Social Provisions Scale, and disease characteristics were assessed by medical record review. General linear models were used to quantify differential gene expression across risk factor groups. Linear regression models were used to examine associations between social isolation and lymphovascular invasion.
Results
Tumors from socially isolated patients showed upregulated expression of genes involved in EMT (average score difference = +0.080 log2 mRNA abundance ± 0.034 standard error) and M2 macrophage polarization (+0.033 ± 0.014) as well as increased density of lymphatic vessels (β= –.29) but no difference in blood vessel density. TELiS promoter–based bioinformatics analyses indicated activation of CREB family transcription factors that mediate the gene-regulatory effects of β-adrenergic signaling (log2 fold-difference in promoter binding site prevalence: mean ± standard error = +0.49 ± 0.19).
Conclusions
Primary breast tumors from socially isolated patients show multiple prometastatic molecular alterations, providing a plausible biological pathway through which poor social support may accelerate breast cancer progression and defining new targets for intervention.
Host characteristics can significantly influence the development and progression of breast cancer (1), and research has increasingly sought to target such effects in order to optimize treatment outcomes and enhance survival. Much research has examined the role of the tumor microenvironment in modulating breast tumor progression and metastasis, including the effects of tumor-associated macrophages (TAMs) (2–5), blood and lymphatic vessels (6, 7), and cell differentiation dynamics such as epithelial–mesenchymal transition (EMT) (8–11). Macrophages are recruited to breast tumors as they develop where they promote angiogenesis, invasion, and metastasis (4). In particular, murine models of breast cancer have demonstrated that alternatively activated or M2 macrophages play an important role in tumor cell migration and invasion (5). TAMs may influence tumor progression by facilitating the EMT, which is critical for tumor cell migration and invasion (8). Macrophages and other inflammatory cells in the tumor microenvironment release EMT-inducing signals, which activate EMT-related transcription factors and associated molecular programs within tumor cells (9, 10). Tumor cells that have acquired mesenchymal attributes show increased invasion, metastasis, and resistance to chemotherapy (11). TAMs may also influence tumor progression through effects on the lymphatic system, which provides a pathway for tumor cells to escape the primary tumor and colonize distant tissue sites. TAMs have been shown to increase lymphatic vessel density (LVD) in preclinical models (12), and tumor-associated LVD is associated with poor prognosis in breast cancer (6, 7).
Tumor progression is also influenced by the broader “tumor macro-environment” of the host’s systemic physiology and the life circumstances that modulate those systemic effects on the biology of tumor cells and their microenvironment (13–15). For example, preclinical mouse models of human cancer have shown that chronic activation of fight-or-flight stress physiology from the sympathetic nervous system (SNS) can promote breast tumor metastasis via β-adrenergic upregulation of multiple prometastatic pathways including TAM recruitment, tumor angiogenesis and lymphangiogenesis, and EMT (12, 16). Stress-induced glucocorticoid signaling from the hypothalamic–pituitary–adrenal axis can also promote tumor development, chemoresistance, and metabolic reprogramming of mammary adipose tissue in ways that promote tumor progression (15, 17). Social isolation of tumor-bearing mice has also been found to accelerate mammary tumor progression (18) and upregulate angiogenesis and TAM recruitment while downregulating tumor-associated T-cell prevalence and activation (19, 20). However, the relevance of these findings to human breast cancer remains unclear because few studies have examined the relationship between patient life circumstances and the molecular biology of their breast tumors.
Epidemiologic analyses have identified social isolation as one of the most robust patient-level risk factors for cancer progression and mortality (21, 22). Socially isolated breast cancer patients show decreased survival in multiple cohort studies (23), including the NCI Black/White Survivors Study (n = 1011) (24), the Nurses’ Health Study (n = 2835) (25), and the After Breast Cancer Pooling Project (n = 9267) (26). Although isolation-related differences in breast cancer survival are well established, the mechanisms underlying this association remain poorly defined and are thus difficult to remedy. Some analyses suggest that differential breast cancer outcomes may stem from differences in health-related behavior and medical treatment in socially isolated women (27). However, results from the preclinical animal models summarized above suggest that stress effects on tumor biology may also contribute. Such effects would be consistent with the increased SNS, HPA-axis, and pro-inflammatory signaling activity observed in humans experiencing social isolation (28–30).
To clarify the biological basis for isolation-related differences in breast cancer mortality, the present analyses examined whether primary tumors from socially isolated breast cancer patients showed increased activity of specific molecular pathways implicated in previous preclinical models of stress effects on breast cancer progression, focusing on upregulation of EMT, M2 macrophage polarization, and blood and lymphatic vessel density (12, 16). Gene expression profiling was used to quantify EMT-related and M1/M2-characteristic gene transcripts, and immunohistochemistry (IHC) was used quantify density of blood and lymphatic vessels in primary breast tumors using antibodies to identify intratumoral vascular (CD31) and lymphatic (LYVE-1) endothelial cells.
Methods
Participants
Fifty-six participants were identified for these analyses from the UCLA RISE study, a prospective observational study of women recently diagnosed with early-stage (0–IIIA) breast cancer. RISE study patients were recruited from physician offices after diagnosis but before onset of adjuvant or neoadjuvant therapy with chemotherapy, radiation, trastuzumab, and/or endocrine therapy. The current study focused on a subgroup of RISE study patients who met the following inclusion criteria: 1) diagnosis with stage I, II, or III breast cancer; 2) resection of primary tumor at UCLA; 3) breast tumor tissue available for analysis; and 4) no neoadjuvant chemotherapy.
Procedures
After enrollment in the RISE study, patients completed self-report questionnaires to assess demographic and psychosocial factors, including social isolation. Formalin-fixed paraffin-embedded (FFPE) tumor blocks were obtained from the UCLA Translational Pathology Core Laboratory (TPCL) and sectioned for analysis. The UCLA Institutional Review Board approved all study procedures, and informed consent was obtained from all patients.
Social Isolation
The attachment subscale of the Social Provisions Scale (SPS) was used to assess subjective experiences of social attachment/isolation (31–33). This four-item scale measures the extent to which an individual feels emotionally connected to vs isolated from others and has good reliability and validity, including predictive, convergent, and discriminant validity (32). The coefficient alpha for the attachment subscale was 0.75 in the validation sample (32) and 0.79 in the current study. The attachment subscale is associated with biological and health outcomes in ovarian cancer (34–37). In line with previous work, social isolation was indicated by an SPS attachment subscale score of 14 or less (possible range = 0–16). Of note, this scale is not specific to social support related to cancer and thus provides a more general measure of perceived social connection/isolation.
Demographic and Clinical Characteristics
Self-report questionnaires were used to assess age, ethnicity, and marital status. Height and weight were measured for determination of body mass index (BMI). Clinical information was abstracted from medical records and reviewed by a medical oncologist (PAG) to determine tumor characteristics (stage, estrogen receptor [ER]/progesterone receptor [PR]/human epidermal growth factor receptor 2 [HER2] status, Ki67%), which were used to define breast cancer subtypes.
Tumor Samples
Laser Microdissection
For in situ analysis of the whole tumor transcriptome, two adjacent 5 μm sections of formalin-fixed paraffin-embedded breast tumors were cut, one onto a positively charged glass slide (Fisher Scientific) for hematoxylin and eosin (H&E) staining and one onto a polyethylene naphthalate membrane glass slide (Leica) for laser-capture microdissection (LCM) using a Leica LMD7000 Laser Microdissection System. A pathologist (RA) mapped the tumor perimeter based on the H&E section, and a 0.55 mm margin was added to that perimeter to define the LCM capture region (to ensure that we captured relevant microenvironmental tissue). Subsections were captured using a 349 nm adjustable pulse laser (pulse frequency 10–5000 Hz; pulse length <4 ns; maximum pulse energy 120 µJ), deparaffinized (Qiagen), and processed for total RNA extraction (Qiagen) with DNase treatment (Qiagen). One sample was not available for LCM.
Gene Expression Profiles
LCM RNA samples were tested for suitable mass (Nanodrop ND1000) and were subject to genome-wide transcriptional profiling using Ambion TotalPrep cRNA hybridized to Illumina HT-12 v4 BeadArrays in the UCLA Neuroscience Genomics Core Laboratory, following the manufacturers’ specified protocols. All assay and normalization procedures were performed blind to participant characteristics. Transcript abundance values were quantile-normalized and log2-transformed before analysis. One sample yielded insufficient target cRNA and was excluded from further analyses.
Immunohistochemistry
The purpose of our IHC studies was to identify and quantify intratumoral blood vessels and lymphatic vessels. To identify the blood vessels, we used an antibody against the platelet endothelial cell adhesion molecule (PECAM-1), also known as cluster of differentiation 31 (CD31), which has been used extensively to identify the endothelial cells lining the microvasculature (38). For lymphatic vessels, we utilized an antibody that recognizes the protein lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), a cell surface receptor on lymphatic endothelial cells that has been previously described as a marker of lymphatic endothelial cells (39). Combining IHC with digital slide scanning allowed us to quantitate the density of vessels within a given section of tumor slides.
IHC was performed on sections of formalin-fixed paraffin-embedded breast tumors adjacent to those used for gene expression profiling. Regions of interest were selected based on the pathologic characteristics (nuclear and cellular morphology) from a hematoxylin-stained section. Primary polyclonal antibodies against CD31 (clone ab28364, 1:100 dilution, ABCAM, Cambridge, MA) and LYVE-1 (clone PA1-16635, 1:200 dilution, Thermo Fisher Scientific, Waltham, MA) were applied to formalin-fixed paraffin-embedded sections. The sections were deparaffinized in xylene and rehydrated through graded alcohols to distilled water before undergoing antigen retrieval. Briefly, antigen retrieval was done by heating in citrate buffer (pH 6.0), followed by blocking with 10% normal donkey serum diluted in phosphate-buffered saline. Primary antibodies against CD31 and LYVE-1 were then applied overnight. The secondary antibody was Biotin-SP Conjugated AffiniPure Donkey Anti-Rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA). Visualization was achieved using the Pierce DAB Substrate Kit (Thermo Fisher Scientific, Waltham, MA).
Digital Slide Scanning and Image Analysis
Whole slides were scanned using the Aperio Digital Pathology Slide Scanner ScanScope AT Turbo (Leica Biosystems, Buffalo Grove, IL). Images were analyzed using quantitative analysis software Aperio ImageScope v12.3.2.8013. The algorithm for microvessel analysis quantitatively assessed the number of vessels and analysis area based on spectral separation of the DAB-stained structures. These structures were then used to compute a measure of microvessel density per unit area (um2). Several samples could not be stained or had insufficient tumor for analysis (n = 7 for LYVE-1, n = 8 for CD31).
Statistical Analyses
General linear models were used to quantify differential gene expression across risk factor groups (high vs low social isolation) while controlling for age, race (white/nonwhite), marital status, BMI, disease stage, and breast tumor subtype. Genomics analyses tested two primary hypotheses involving 1) a possible isolation-related difference in average expression of 130 EMT-related transcripts previously found to discriminate between epithelial- and mesenchymal-polarized breast cancer cells (NCBI Gene Expression Omnibus GSE13915) (40) and 2) a possible isolation-related difference in average expression of 113 transcripts previously found to discriminate between M1 and M2 macrophage phenotypes (GSE5099) (41). For each primary hypothesis, gene-specific differential expression estimates were combined into a composite contrast score, with the EMT contrast weighting mesenchymal genes +1 and epithelial genes –1, and the M1/M2 contrast weighting M2-characteristic genes +1 and M1-characteristic genes –1. Standard errors for these differential expression contrasts were derived from bootstrap resampling of linear model residual vectors across all genes analyzed, and thus accounted for any potential correlation among residuals across genes. These gene set–based analyses were the only primary hypotheses tested, and no attempt was made to quantify the statistical significance of differential gene expression at the level of individual transcripts, as this study was neither intended to nor powered for individual gene-specific analyses.
Following significant results from primary analyses, ancillary secondary analyses were conducted to test two additional a priori–specified hypotheses regarding specific stress-related neuroendocrine signaling pathways that might potentially contribute to observed differences in EMT and/or M1/M2 gene expression. These analyses were conducted using the TELiS promoter–based analysis of transcription factor–binding motif prevalence (42) applied to consensus human genome sequences for the core promoter regions of all genes showing a 1.2-fold or greater difference in (adjusted) average expression in tumors from patients with low vs high social isolation. Differential activation of CREB family transcription factors (which mediate β-adrenergic signaling from the sympathetic nervous system) was assessed using the TRANSFAC V$CREB_01 position-specific weight matrix, and differential activity of the glucocorticoid receptor (which mediates signaling by glucocorticoid hormones from the hypothalamus–pituitary–adrenal axis) was assessed using the TRANSFAC V$GR_Q6 matrix. In each analysis, the ratio of response element frequencies in the promoters of upregulated vs downregulated genes was taken as a measure of differential activity of transcription control pathways, and (log) ratios were averaged over nine different parametric combinations of promoter length (–300, –600, and –1000 to +200 bp upstream of RefSeq-designated transcription start site) and motif detection stringency (TRANSFAC mat_sim values of 0.80, 0.90, and 0.95) to ensure robust results (42).
Linear regression models were used to examine the association between social isolation and lymphovascular invasion (SPSS, version 24). These analyses used continuous scores on the SPS attachment scale and controlled for age, race (white/nonwhite), marital status, BMI, disease stage, and breast tumor subtype. The outcomes of interest were CD31 microvessel density and LYVE-1 microvessel density.
Results
Participant Characteristics
Fifty-six women met eligibility criteria and were included in the analysis sample. As shown in the first column of Table 1, the majority were white and married, with body mass index in the overweight range. Most had either stage I or stage II breast cancer, and the majority were classified as luminal A. On average, there was a one-month interval between diagnosis and surgical resection of the primary tumor (mean time from diagnosis to surgery = 38.2 days, range = 15–84 days) and a two-month interval between diagnosis and study enrollment (mean time from diagnosis to study enrollment = 69.6 days, range = 30–128 days). The mean score on the SPS attachment scale was 15.16, which is higher than the mean score in the validation sample (mean = 13.72, SD = 2.42) (32) but comparable to the median score in a sample of women recently diagnosed with ovarian cancer (median = 15) (34). Fifteen women (27%) were classified as showing a moderate to high degree of social isolation (SPS attachment ≤ 14). Table 1 also presents information on women classified as high vs low social isolation based on their SPS attachment scores. Women in the high-isolation group had significantly higher BMI and were less likely to be married (P < .05). In addition, there was a significant association between SPS attachment group and molecular subtype (P = .02); women in the high-isolation group had fewer luminal B and more HER2neu-enriched tumors than the low-isolation group. Of note, there was no association between SPS attachment and tumor stage or time from diagnosis to surgery/study enrollment.
Table 1.
Patient characteristics
| All patients (n = 56) | High isolation (n = 15) | Low isolation (n = 41) | |
|---|---|---|---|
| Age, M (SD), y | 57.8 (12.7) | 58.4 (11.1) | 57.6 (13.4) |
| Marital status, No. (%)* | |||
| Married/living as married | 33 (58.9) | 5 (33.3) | 28 (68.3) |
| Not married | 23 (41.1) | 10 (66.7) | 13 (31.7) |
| Race/ethnicity, No. (%) | |||
| White | 42 (75) | 9 (60) | 33 (80.5) |
| Nonwhite | 14 (25) | 6 (40) | 8 (19.5) |
| Body mass index, M (SD)*, kg/m2 | 26.5 (6.7) | 29.8 (7.3) | 25.2 (6.2) |
| Stage, No. (%) | |||
| Stage I | 26 (46.4) | 8 (53.3) | 18 (43.9) |
| Stage II | 28 (50) | 6 (40.0) | 22 (53.7) |
| Stage III | 2 (3.6) | 1 (6.7) | 1 (2.4) |
| Breast tumor subtype, No. (%)* | |||
| Luminal A | 29 (51.8) | 8 (53.3) | 21 (51.2) |
| Luminal B | 16 (28.6) | 2 (13.3) | 14 (34.1) |
| HER2neu enriched | 3 (5.4) | 3 (20.0) | 0 (0) |
| Triple negative | 8 (14.3) | 2 (13.3) | 6 (14.6) |
| Days from diagnosis to surgery, M (SD) | 38.2 (17.6) | 37.2 (17.7) | 38.6 (17.7) |
| Days from diagnosis to study enrollment, M (SD) | 69.6 (24.6) | 66.6 (25.4) | 70.7 (24.5) |
Social Isolation and Gene Expression
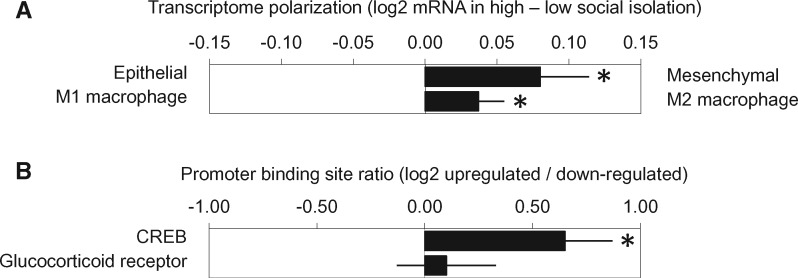
Primary functional genomics analyses examined whether average expression of an a priori–defined set of 130 EMT-related gene transcripts (40) differed as a function of social isolation after control for any potential confounding by age, race, marital status, BMI, tumor stage, and breast tumor molecular subtype. Results indicated increased mesenchymal polarization in tumors from socially isolated patients (average score difference = +0.080 log2 mRNA abundance ± 0.034 standard error, P = .020) (Figure 1A). In separate follow-up analyses of the 67 mesenchymal-characteristic genes and the 63 epithelial-characteristic genes, tumors from isolated patients showed increased expression of the mesenchymal gene composite (+0.188 ± 0.066, P = .006) but no difference in the epithelial gene composite (+0.036 ± 0.035, P = .310). Analyses of M1 and M2 macrophage-related transcripts indicated greater M2 polarity in tumors from socially isolated patients (+0.033 ± 0.014, P = .015) (Figure 1A), controlling for age, race, marital status, BMI, tumor stage, and breast tumor molecular subtype. Similar results emerged when social isolation was represented as a continuous variable (EMT: –0.032 ± 0.015 log2 mRNA abundance/SD increase in SPS attachment scores, P = .032; M1/M2: +0.016 ± 0.008, P = .060).
Figure 1.
A) Analyses of epithelial–mesenchymal transition–related gene transcripts in primary breast tumors from women with early-stage breast cancer indicated increased mesenchymal polarization in tumors from socially isolated patients. *P =.020. Analyses of M1 and M2 macrophage transcripts showed greater M2 polarity in tumors from socially isolated patients. *P =.015. B) Promoter-based bioinformatics analyses indicated increased activity of CREB family transcription factors in tumors from socially isolated patients but no differences in glucocorticoid receptor activity. *P =.012. P values (two-sided) were calculated using .
Ancillary analyses were conducted to identify neuroendocrine signaling pathways that might contribute to differences in gene expression in socially isolated women. Transcriptome profiling identified 534 genes showing greater than 1.2-fold upregulation in tumors from socially isolated patients and 334 genes showing equivalent downregulation. TELiS analyses of transcription factor binding motif prevalence in the promoters of differentially expressed genes implicated increased CREB activity in driving the observed transcriptome differences (log2 fold-difference in promoter binding site prevalence: mean = +0.49 ± 0.19, P = .012) (Figure 1B) but showed no indication of differential glucocorticoid receptor activity (+0.27 ± 0.26, P = .298), controlling for demographic and disease-related characteristics.
Social Isolation and Lymphatic and Vascular Density
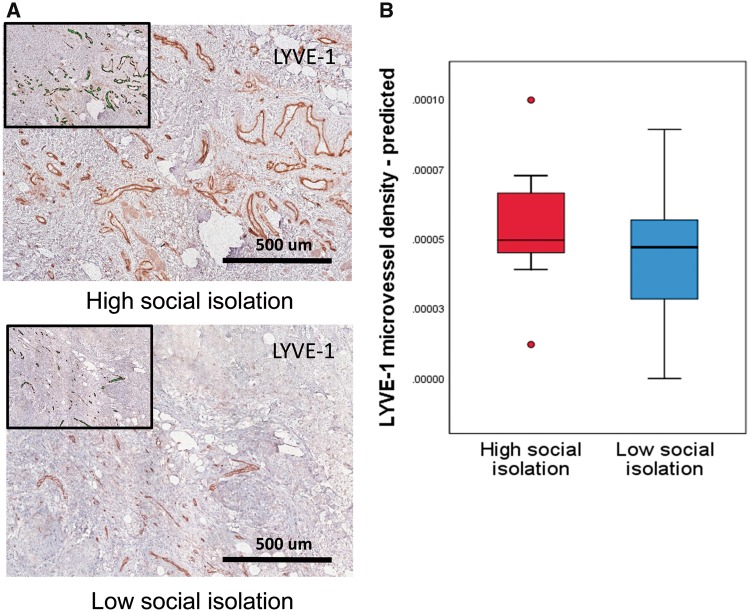
Tumors from socially isolated patients had greater intratumoral LYVE-1 microvessel density, controlling for age, race, marital status, BMI, tumor stage, and breast tumor molecular subtype (β = –0.29, P = .050). Figure 2A shows representative examples in specimens from patients high and low on social isolation, and boxplots of predicted scores on LYVE-1 microvessel density in the high– and low–social isolation groups are shown in Figure 2B. There was no association between social isolation and CD31 microvessel density (P > .70).
Figure 2.
Analyses of lymphovascular invasion in primary breast tumors indicated increased intratumoral lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) microvessel density in tumors from socially isolated women. A) Representative images of LYVE-1-stained sections from patients reporting high vs low social isolation. B) Box plots of predicted scores for LYVE-1 microvessel density in the high– and low–social isolation groups. Each box indicates the interquartile range, and the middle line indicates the median for that group. LYVE-1 = lymphatic vessel endothelial hyaluronan receptor 1.
Association Between Gene Expression and Lymphovascular Density
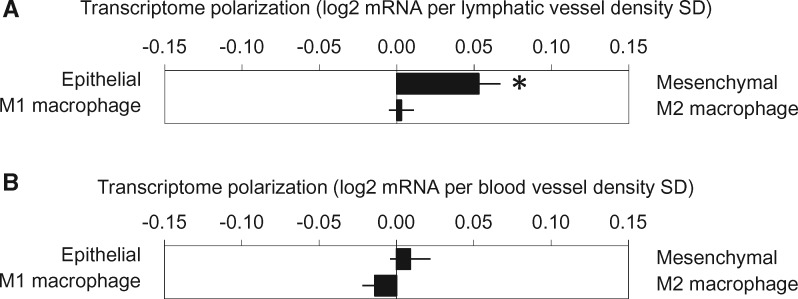
Analyses relating LVD and CD31 density to gene expression (controlling for demographic and tumor-related covariates as above) linked LVD to greater EMT polarization (+0.041 ± 0.016 log2 RNA units per SD of LVD, P = .012) (Figure 3A), but no difference in M1/M2 polarization was observed (+0.006 ± 0.008, P = .463). Neither EMT nor M1/M2 transcriptome bias correlated with CD31 microvessel density (+0.008 ± 0.014, P = .578, and 0.004 ± 0.006, P = .556, respectively) (Figure 3B).
Figure 3.
A) Lymphatic microvessel density was associated with increased epithelial–mesenchymal transition (EMT) polarization but no difference in M1/M2 polarization. *P =.012, two-sided. B) Vascular microvessel density was not associated with EMT or M1/MT transcriptome bias.
Discussion
These analyses document systematic upregulation of multiple prometastatic molecular processes in primary breast tumors from socially isolated breast cancer patients. Social isolation was associated with upregulation of large sets of genes involved in EMT and M2 macrophage polarization as well as increased density of lymphatic vessels in the primary tumor and its microenvironment. These processes are associated with enhanced tumor cell migration, invasion, and metastasis (3–8,11). Of note, results were independent of patient age, race, marital status, BMI, disease stage, and tumor subtype. Consistent with a potential contribution from systemic neuroendocrine influences, TELiS promoter–based bioinformatic analysis of gene regulatory pathway activity also linked social isolation to increased signaling from the CREB family transcription factors that mediate the gene-regulatory effects of β-adrenergic signaling from the SNS. These findings are highly consistent with findings from preclinical mouse models linking chronic stress–induced SNS activation to EMT, M2 polarization, and lymphangiogenesis in primary breast tumors (12,16) and with findings from previous studies documenting increased SNS activity and pro-inflammatory gene activation in socially isolated humans (28,29). These results suggest that epidemiologic indications of reduced cancer survival in socially isolated breast cancer patients (21,26) may stem at least in part from host physiological influences on tumor cell biology and the surrounding microenvironmental processes that regulate breast cancer progression.
Although the present findings are broadly consistent with the molecular mechanisms observed in preclinical animal models of human cancer, we found no evidence that primary breast tumors from socially isolated women showed increased blood vessel invasion or indications of upregulated glucocorticoid receptor signaling. Both of these dynamics have been observed in previous preclinical cancer models in which animals are exposed to chronic stress (18,43). These differences may be due to differences in the nature or chronicity of the stressor or in the tumor model.
Limitations of this study include the relatively homogeneous sample, which was comprised primarily of ER+/PR+/HER2- luminal A breast tumors and thus may not be generalizable to tumors with other characteristics. In particular, triple-negative tumors were not well represented in this sample, although neuroendocrine signaling through the sympathetic nervous system may be particularly relevant for outcomes in this group (44,45). The examination of psychosocial risk factors in the different biological subtypes of breast cancer will facilitate the identification of vulnerable patients who may benefit from targeted therapies. The present results come from a cross-sectional analysis and thus cannot definitively support a causal effect of social isolation on the observed molecular outcomes. It is conceivable, for example, that part of the association observed here may stem from more aggressive tumors inducing changes in the brain processes underlying social behavior (eg, via effects of tumor-derived cytokines [46,47]). The clinical significance of the observed molecular differences also remains to be defined in future research.
From a clinical perspective, the molecular pathways identified in this report represent potential targets for interventions to protect patients against the adverse biological effects of social isolation in the highly stressful context of breast cancer. A recent phase II trial with early-stage breast cancer patients demonstrated beneficial effects of perioperative COX-2 and β-adrenergic blockade on EMT gene expression and monocyte infiltration in primary breast tumors (48), both of which were linked with social isolation in this study. Thus, treatments that target β-adrenergic and/or inflammatory signaling pathways could potentially reduce the negative effects of social isolation on tumor biology and ultimately help enhance clinical outcomes. Future research will be required to directly quantify the clinical impact of targeting social isolation, but such effects would be consistent with data from several previous randomized controlled intervention studies that document reduced breast cancer progression and mortality in patients treated with multicomponent lifestyle interventions that target stress reduction and enhanced social support (49,50). In the context of future clinical trials, the present data identify multiple molecular biomarkers that could be used to assess the biological impact of reducing social isolation in breast cancer patients as they confront this highly significant health threat.
Funding
This work was supported by the Breast Cancer Research Foundation (BCRF), the National Institutes of Health (R01 5R01CA160427), and the UCLA Advanced Light Microscopy and Spectroscopy Laboratory. DML was supported by a Career Development Award from the National Cancer Institute (K07 CA188237).
Notes
Affiliations of authors: Department of Psychology (JEB), Department of Psychiatry and Biobehavioral Sciences (JEB, DML, SWC), Department of Pathology and Laboratory Medicine (PS), Department of Medicine (JA, SWC), School of Medicine (PAG), and School of Public Health (PAG), UCLA, Los Angeles, CA; Cousins Center for Psychoneuroimmunology (JEB, DML, SWC), UCLA, Los Angeles, CA; Jonsson Comprehensive Cancer Center (JEB, DML, SWC, PAG), UCLA, Los Angeles, CA; Department of Radiation Oncology (SLS, FM), Department of Biomedical Sciences (SLS), Department of Surgery (FM), and Samuel Oschin Comprehensive Cancer Institute (AA), Cedars-Sinai Medical Center, Los Angeles, CA; Kaiser Permanente Medical Center (RA), San Jose, CA.
Conflicts of interet: PAG is a member of the scientific advisory board of the BCRF.
References
- 1. Goodwin PJ, Meyerhardt JA, Hursting SD.. Host factors and cancer outcome. J Clin Oncol. 2010;2826:4019–4021. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Coussens LM.. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;213:309–322. [DOI] [PubMed] [Google Scholar]
- 3. Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Letters. 2013;3321:3–10. [DOI] [PubMed] [Google Scholar]
- 4. Qian BZ, Pollard JW.. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;1411:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams CB, Yeh ES, Soloff AC.. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamura Y, Yasuoka H, Tsujimoto M et al. , . Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;912:125–132. [DOI] [PubMed] [Google Scholar]
- 7. Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN.. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;202:183–191. [DOI] [PubMed] [Google Scholar]
- 8. Thiery JP, Acloque H, Huang RY, Nieto MA.. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;1395:871–890. [DOI] [PubMed] [Google Scholar]
- 9. Chaffer CL, Weinberg RA.. A perspective on cancer cell metastasis. Science. 2011;3316024:1559–1564. [DOI] [PubMed] [Google Scholar]
- 10. Su S, Liu Q, Chen J et al. , . A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;255:605–620. [DOI] [PubMed] [Google Scholar]
- 11. Kalluri R, Weinberg RA.. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;1196:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le CP, Nowell CJ, Kim-Fuchs C et al. , . Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antoni MH, Lutgendorf SK, Cole SW et al. , . The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 2006;63:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK.. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;159:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volden PA, Conzen SD.. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30(Suppl):S26–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sloan EK, Priceman SJ, Cox BF et al. , . The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;7018:7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volden PA, Wonder EL, Skor MN et al. , . Chronic social isolation is associated with metabolic gene expression changes specific to mammary adipose tissue. Cancer Prev Res (Phila). 2013;67:634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hermes GL, Delgado B, Tretiakova M et al. , . Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci U S A. 2009;10652:22393–22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Budiu RA, Vlad AM, Nazario L et al. , . Restraint and social isolation stressors differentially regulate adaptive immunity and tumor angiogenesis in a breast cancer mouse model. Cancer Clin Oncol. 2016;61:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madden KS, Szpunar MJ, Brown EB.. Early impact of social isolation and breast tumor progression in mice. Brain Behav Immun. 2013;30:S135–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinquart M, Duberstein PR.. Associations of social networks with cancer mortality: A meta-analysis. Crit Rev Oncol Hematol. 2010;752:122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aizer AA, Chen M-H, McCarthy EP et al. , . Marital status and survival in patients with cancer. J Clin Oncol. 2013;3131:3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC.. Breast cancer and social environment: Getting by with a little help from our friends. Breast Cancer Res. 2016;181:54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds P, Boyd PT, Blacklow RS et al. , . The relationship between social ties and survival among black and white breast cancer patients. National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1994;33:253–259. [PubMed] [Google Scholar]
- 25. Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I.. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;247:1105–1111. [DOI] [PubMed] [Google Scholar]
- 26. Kroenke CH, Michael YL, Poole EM et al. , . Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer. 2017;1237:1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kroenke CH, Michael YL, Shu X-O et al. , . Post-diagnosis social networks, and lifestyle and treatment factors in the After Breast Cancer Pooling Project. Psychooncology. 2017;264:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT.. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;1087:3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cole SW, Capitanio JP, Chun K et al. , . Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci U S A. 2015;11249:15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eisenberger NI, Cole SW.. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;155:669–674. [DOI] [PubMed] [Google Scholar]
- 31. Cutrona CE. Social support and stress in the transition to parenthood. J Abnorm Psychol. 1984;934:378–390. [DOI] [PubMed] [Google Scholar]
- 32. Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. In: Jones WH, Perlman D, eds. Advances in Personal Relationships. Greenwich, CT: JAI Press; 1987:37–67. [Google Scholar]
- 33. Russell D, Cutrona CE, Rose J, Yurko K.. Social and emotional loneliness: An examination of Weiss's typology of loneliness. J Pers Soc Psychol. 1984;466:1313–1321. [DOI] [PubMed] [Google Scholar]
- 34. Lutgendorf SK, De Geest K, Bender D et al. , . Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;3023:2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lutgendorf SK, Lamkin DM, Jennings NB et al. , . Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;1421:6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lutgendorf SK, Sood AK, Anderson B et al. , . Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;2328:7105–7113. [DOI] [PubMed] [Google Scholar]
- 37. Costanzo ES, Lutgendorf SK, Sood AK et al. , . Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;1042:305–313. [DOI] [PubMed] [Google Scholar]
- 38. Strilic B, Yang L, Albarrán-Juárez J et al. , . Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;5367615:215–218. [DOI] [PubMed] [Google Scholar]
- 39. Padera TP. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;2965574:1883–1886. [DOI] [PubMed] [Google Scholar]
- 40. Choi Y-L, Bocanegra M, Kwon MJ et al. , . LYN is a mediator of epithelial-mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;706:2296–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinez FO, Gordon S, Locati M, Mantovani A.. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;17710:7303–7311. [DOI] [PubMed] [Google Scholar]
- 42. Cole SW, Yan W, Galic Z, Arevalo J, Zack JA.. Expression-based monitoring of transcription factor activity: The TELiS database. Bioinformatics. 2005;216:803–810. [DOI] [PubMed] [Google Scholar]
- 43. Thaker PH, Han LY, Kamat AA et al. , . Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;128:939–944. [DOI] [PubMed] [Google Scholar]
- 44. Botteri E, Munzone E, Rotmensz N et al. , . Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013;1403:567–575. [DOI] [PubMed] [Google Scholar]
- 45. Melhem-Bertrandt A, Chavez-Macgregor M, Lei X et al. , . Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;2919:2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW.. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;91:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ.. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A. 2009;10622:9069–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shaashua L, et al. , . Perioperative COX-2 and beta-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;2316:4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andersen BL, Yang H-C, Farrar WB et al. , . Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;11312:3450–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stagl JM, Lechner SC, Carver CS et al. , . A randomized controlled trial of cognitive-behavioral stress management in breast cancer: Survival and recurrence at 11-year follow-up. Breast Cancer Res Treat. 2015;1542:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]