Table 1. Optimization of the reduction mediated by Co(acac)2 a .
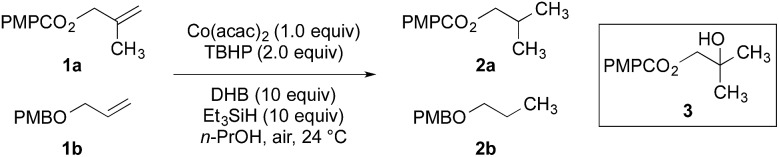
| |||||||
| Entry | Variation from above | Time | Conv. 1a | Yield 2a | Conv. 1b | Yield 2b | 2a : 2b |
| 1 | None | 135 min | >95% | 71% | 14% | 14% | 5.1 : 1.0 |
| 2 | 0 °C | 300 min | <5% | <1% | 7% | <1% | — b |
| 3 | Co(acac)2, TBHP (50 mol% each) | 180 min | 72% | 31% | 18% | <1% | — b |
| 4 | Open flask | 30 min | >95% | 63% | 56% | 17% | 3.7 : 1.0 |
| 5 | Co(acac)2, TBHP (25 mol% each), open flask | 180 min | 75% | 25% (69% of 3) c | 6% | <1% | — b |
| 6 | Argon | 360 min | 81% | 69% | 33% | 14% | 4.9 : 1.0 |
| 7 | Argon, 50 °C | 120 min | >95% | 80% | 25% | 18% | 4.4 : 1.0 |
| 8 | TBHP (1.0 equiv., slow addition), argon, 40 °C | 60 min | >95% | 91% | 28% | 20% | 4.6 : 1.0 |
aReactions employed 250 μmol each of 1a and 1b. Conversions and yields were determined by 1H NMR spectroscopy using mesitylene or 1,3,5-trimethoxybenzene as an internal standard.
bThe ratio of 2a : 2b could not be determined due to the absence of 2a and/or 2b in the 1H NMR spectrum of the unpurified product mixture.
c69% of 3 was isolated after purification by flash-column chromatography.
