Table 3. Scope of aromatic and alkyl substituents.

| |||
| Entry | Starting material | Product | Yield a |
| 1 |

|

|
90% (1 h) |
| 2 |

|

|
84% (1 h) |
| 3 |

|

|
88% (1 h) |
| 4 |

|

|
90% (1 h) |
| 5 |

|

|
93% (1 h) |
| 6 |

|

|
31% b (1 h) |
| 7 |

|

|
80% (1 h) |
| 8 |

|

|
80% (18 h) |
| 9 |

|

|
46% c , d 3 : 2 rr e (26 h) |
| 10 |

|

|
65% c 1 : 1 rr e (22 h) |
| 11 |

|

|
88% (1 h) |
| 12 |
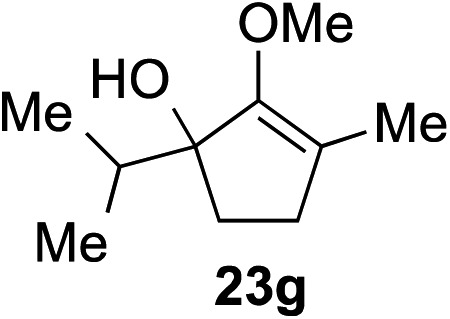
|

|
80% c 3 : 1 rr e (1 h) |
| 13 |

|

|
78% c 4 : 1 rr e (1 h) |
aIsolated yield of products 26 after flash chromatography.
bThe low yield was attributed to poor solubility of the product in most organic solvents.
cCombined yield for both regioisomers.
dThe major regioisomer was not assigned, as these compounds were not separable by chromatography.
eThe ratio of regioisomers was determined by 1H NMR of the crude reaction mixture.
