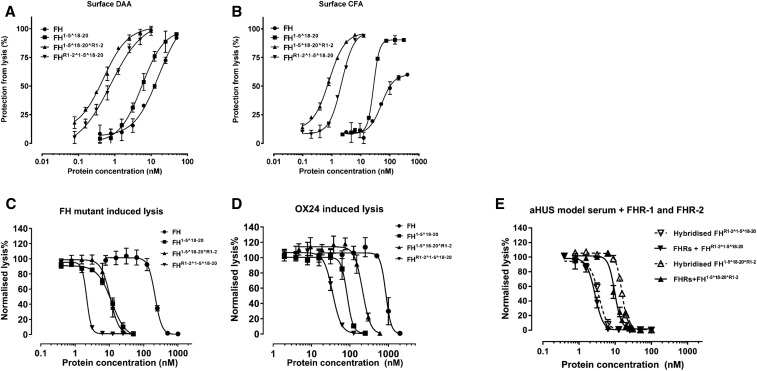
Figure 4.
HDM-FH protects “host-Like” surfaces significantly better than FH. (A) Decay acceleration activity of FH reagents on surface-bound AP C3 convertase. C3 convertases were reconstituted on the surface of C3b as described in the Methods. The decay of the AP C3 convertases was accelerated by FH (●), FH1–5^18–20 (▪), FHR1–2^1–5^18–20 (▼), and FH1–5^18–20^R1–2 (▲). The remaining AP C3 convertase activity was then determined by the amount of lysis of sheep erythrocyte after incubation with FB- and FH-depleted serum in PBS supplemented with 20 mM EDTA. (B) FI CFA of FH and its derivatives. The C3b precoated sheep erythrocytes were exposed to a concentration gradient of FH reagents together with 2.5 μg/ml of FI. The remaining C3b molecules on the sheep erythrocyte surface bind FB (70 μg/ml) and FD (0.4 μg/ml) to form AP C3 convertase, which leads to cell lysis during incubation with FB- and FH-depleted serum in PBS supplemented with 20 mM EDTA. Plasma purified FH, FH1–5^18–20, FHR1–2^1–5^18–20, and FH1–5^18–20^R1–2 also protect sheep erythrocytes from lysis in human sera with deregulated complement AP. (C) The addition of increasing concentrations of FH reagents prevented sheep erythrocyte lysis in FH-depleted serum supplemented with recombinant human FH S1191A V1197L.27 (D) The increasing concentrations of FH reagent, as indicated, protected sheep erythrocytes from AP-mediated lysis in OX24-spiked normal human serum (an autoantibody model serum23). (E) The functional effects of potential heterodimer formation between homodimeric mini-FH and recombinant FHR were evaluated using a hemolytic assay essentially as described above. However, before serum was added, a concentration series of doubly diluted compounds, as indicated, were preincubated with either recombinant human FHR-1 and FHR-2 at physiologic serum concentration (solid line) or buffer alone (dashed line) for 1 hour. Sheep erythrocyte lysis was measured by the hemoglobin release (A405). For (A) and (B), data were processed using 100% lysis determined in water and 0% lysis of diluted serum diluted in buffer alone, whereas for (C) and (D) the data were normalized against sheep erythrocyte lysis with deregulated sera in the absence of FH reagent. Six independent measurements of three experiments were shown, except for (A) and (B) that show data from three independent measurements of one experiment. The mean and SD for each measurement were calculated for all datasets; sigmoidal curves were fitted using nonlinear regression function in GraphPad Prism. aHUS, atypical Haemolytic ureamic syndrome; DAA, decay accelerating activity; CFA, cofactor activity.