Abstract
Prior research indicates that cognitive vulnerabilities can render individuals more susceptible to psychopathology in the wake of stressful events. However, little work has directly targeted the neural mechanisms involved. In this study, we examined fMRI activity as a function of negative cognitive style, a well-studied cognitive vulnerability for depression. We adapted a robust paradigm in which undergraduate students completed fMRI testing following a known ecologically-valid stressor (a midterm exam). Negative cognitive style correlated with brain activity in response to both negative and exam-related information in dorsolateral prefrontal cortex and/or angular gyrus, both regions involved in abstract, self-referential thought. There were commonalities and differences in patterns of activity, suggesting that these individuals may process domain-general and domain-specific negative information in different ways but drawing upon a common frontoparietal network. This study thus identifies a potential brain network associated with negative cognitive style, and enhances our understanding of neural mechanisms of cognitive vulnerability to psychopathology.
Keywords: fmri, cognitive vulnerability, stress, depression
A wealth of evidence has established that stress plays a causal role in development of psychopathology (e.g., Belsky & Pluess, 2009; Hammen, 2005; Herringa et al., 2013). However, not all individuals are equally susceptible. Instead, strong support exists for diathesis-stress models, in which negative events interact with pre-existing vulnerabilities to confer risk (Belsky & Pluess, 2009). In particular, cognitive vulnerabilities have been well-studied, and numerous such vulnerabilities have been identified, such as neuroticism (Kendler, Gatz, Gardner, & Pedersen, 2006), negative cognitive style (Abramson et al., 2002), and trait rumination (Nolen-Hoeksema, 2000).
While such research is ongoing, there has been an explosion of interest in the pathophysiology of psychiatric disorders, and much has been learned about neural circuitry thought to instantiate them (for examples of recent reviews, see: Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015; Tovote, Fadok, & Lüthi, 2015). However, relatively little is known about the neural mechanisms involved in cognitive vulnerabilities. Recently, there have been calls to understand core biological and psychological processes in psychiatric disorders, most notably the NIMH’s RDoC initiative (Kozak & Cuthbert, 2016). Neuroimaging studies of cognitive vulnerabilities fit well within the RDoC schema as they stand to inform not only our understanding of these established core constructs themselves, but of how brain networks which have recently been elaborated might respond under stress.
Among the most well-supported diathesis stress models is the Hopelessness Theory, which posits a cognitive vulnerability termed negative cognitive style (NCS; Abramson, Metalsky, & Alloy, 1989). Individuals with NCS have a tendency to attribute negative events to stable, global causes with negative consequences and reflective of negative self-characteristics. Such individuals are demonstrably more vulnerable to developing depression (Abramson et al., 1999; Alloy et al., 2006; Hankin, Abramson, Miller, & Haeffel, 2004; Hankin, Abramson, & Siler, 2001) and have higher lifetime rates of depression (Alloy et al., 2000). The midterm exam study is a paradigm that has been used extensively to study NCS in the context of a predictable stressor. In this design, undergraduate students in a course are tested for cognitive style early in the semester, and their self-reported negative affect is followed after an exam. Students with NCS show elevated negative affect compared to their low-risk counterparts for at least a week following a poor midterm grade (Haeffel, 2011; Metalsky, Halberstadt, & Abramson, 1987; Metalsky, Joiner, Hardin, & Abramson, 1993). This post-exam period thus provides a window within which psychological processes reliably differ as a function of NCS—an appealing target for studies of cognitive and neural mechanisms. The present study thus sought to capitalize on this window using neuroimaging.
Our goal was to deploy fMRI to assess how individuals varying in NCS differentially processed information directly related to a recent stressor, i.e., the midterm. Accomplishing this aim required a cognitive task which would cue individuals to recall the midterm and, ideally, allow them to process these memories naturally. For this, we adapted a previously-published task in which participants rehearse the order of words in working memory (Joormann, Levens, & Gotlib, 2011). Notably, the semantic content of the words in this task is irrelevant to task instructions, so errors in performance or differences in brain activity are presumed to be caused by task-unrelated processing triggered by those words. The task was adapted to include two types of stimuli. The first were negative stimuli, which have been widely used to study affective processing (e.g., Hamilton et al., 2012). The second were exam-specific stimuli, i.e., words from exam study materials. These words are not a priori negative, but they are directly relevant to the recent event. We predicted that both negative and exam-related words would induce differential processing in individuals with NCS, and these would be detectable by increases in fMRI activity and deficits in task performance.
By including both stimulus types, we could glean additional nuance by comparing domain-general (negative) stimuli to domain-specific (exam-related) stress-relevant stimuli. It has been previously theorized that maladaptive attributions frame negative events in terms of self-relevant goals, thus making it harder to disengage attention and causing rumination (Abramson et al., 2002). Furthermore, previous work has found that rumination mediates the relationship between negative cognitive style and depressive symptoms after a negative event (Spasojević & Alloy, 2001). Thus, we predicted that stress-specific (i.e., exam-related) words would more reliably alter cognition, i.e., induce more errors and more fMRI activity, as they would trigger self-relevant goals and maladaptive attributions related to the midterm. Although self-reported state rumination was not measured out of concern for task interference, we collected electrodermal activity (EDA), a marker of sympathetic arousal which can index emotional reactivity, attention and cognitive load. EDA is not specific to rumination, but could provide supporting evidence.
Here we used fMRI which captures the blood oxygen-level dependent (BOLD) signal, an indirect measure of neural function. Our analyses were conducted across the entire brain, without a priori tests for specific regions, but we predicted involvement of several networks which have been implicated in depression and whose functions are relevant to NCS. The first of these is the default-mode network (DMN), a network known to be active at rest and thought to play an important role in self-referential thought and autobiographical memory (Raichle, 2015). Depression is associated with resting-state hyperconnectivity of the DMN (Kaiser et al., 2015) and a failure to downregulate DMN during cognitive tasks (Grimm et al., 2008, 2009; Sheline et al., 2009). These findings suggest that it is tonically active in depressed individuals, perhaps due to rumination (Berman et al., 2011; Hamilton et al., 2011). As the maladaptive causal attributions of NCS are also abstract and self-referential, we suspected the DMN would be important in implementing these. A second network of interest was the frontoparietal network (FPN), thought to be critical for cognitive control (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). Prior research indicates that the FPN can couple with the DMN when participants engage in self-referential, goal-directed thinking (Spreng & Schacter, 2012; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). We reasoned that the FPN would be involved in directing cognition towards self-relevant negative processing such as rumination on causal attributions or negative schemas. Finally, we predicted differences in task-evoked amygdala activity. Enhanced amygdala reactivity to emotional stimuli is well-documented in depression (Jaworska, Yang, Knott, & MacQueen, 2015), and one prior study has found amygdala hyperactivity to emotional stimuli in students with NCS (Zhong et al., 2011). Of interest was whether amygdala reactivity in response to negative and task-related stimuli would show similarities; if so, this would suggest that the latter were being interpreted as negative stimuli despite being objectively neutral.
In summary, we recruited undergraduate students varying in negative cognitive style for neuroimaging following a midterm exam. We note that this is not a clinical sample; although these students carried a known risk factor for depression, they were not currently depressed at the time of the study. Our aim was to study a “pure” cognitive vulnerability, i.e., in the absence of concurrent symptomatology. In order to isolate our population of interest—students who had experienced a negative event—we furthermore excluded individuals who received an A and included only participants who performed at or below their desired exam grade.
Our primary questions of interest were as follows:
Do individuals with NCS show deficits in cognitive processing or increases in brain activity in response to stress-relevant information?
Are these differences greater for domain-general (negative) information, domain-specific (exam-related) information, or both?
Method
Participants
Participants for this study were students enrolled in an introductory psychology course (Psychology 202) at UW–Madison in the Fall of 2016. 323 students completed an online questionnaire. 120 of these students then completed a second, in-person questionnaire session. Finally, 60 students completed an MRI session after either the first or second midterm exam (first exam N=27, second exam N=33). Of these, 46 (77%) were female, 55 (92%) were white, and 55 (92%) were non-Hispanic. Average age was 18.59 (SD = 1.42, range 18–28). Fifty-six participants (93%) performed one-half or more letter grades below their aspiration for the midterm, and four (7%) received their desired exam grade. Participation in the study was limited to US citizens, for payment reasons. After the initial online survey, students were excluded from further participation if they endorsed a current or recent (within six months) diagnosis of depression, or use of psychiatric medications during that time period. Students were excluded from the MRI session if they were ineligible for MRI scanning due to metal in their bodies, neurological conditions or claustrophobia.
Procedure
This study had three participation arms. The first comprised an online survey, which students completed at home at their leisure. Second, students who completed the online survey were invited for an in-person questionnaire session. These sessions took place throughout approximately the first half of the semester, in order to screen an adequately-sized pool of participants for MRI recruitment. Following a midterm exam, students who had completed both sessions and who scored at or below their self-reported aspiration on the exam were contacted by phone and screened for MRI eligibility. MRI sessions were conducted after the first and second midterms, but each student could participate only once. Students were scanned within one week of the receipt of exam grades, which was 2–5 days after the exam itself. Hence, students were scanned in the window 7–14 days (M=10.55 days) after the exam date.
Questionnaires
An online survey was used to assess study inclusion criteria. Participants were asked: if they had a current or prior (6 month) diagnosis of depression; if they were currently or had recently (6 months) taken antidepressant medications; their desired exam grades, their high school GPA and their scores on the ACT. Students also completed the Beck Depression Inventory–II (BDI-II; Beck, Steer, & Brown, 1996, Cronbach's alpha1 = 0.91).
In the in-person survey session, participants completed the Cognitive Styles Questionnaire (CSQ; Haeffel et al., 2008), a validated instrument including 24 scenarios comprising positive, negative, achievement-related and social events (i.e., 8 positive social, 8 negative achievement, and so forth). Participants read each scenario (e.g., being unable to complete coursework for an important class) and write down one cause of that event, then respond to one question each on the following: internality, globality and stability of the event’s cause, anticipated negative consequences and impact on self-worth (the event’s meaning), and finally the importance of the event. The five subscales internality, globality, stability, negative consequences and self-worth are averaged within and then between each of the four scenario types in order to generate scores for, e.g., negative cognitive style in the achievement domain. These are further averaged into overall negative and positive cognitive styles. All scales are reported on a 7-point scale where 7 indicates the most negative (or positive) cognitive style. Hereafter we refer to the negative cognitive style score as the CSQ total score, which had alpha = 0.93. We were also interested specifically in the CSQ achievement subscale, which was the negative cognitive style calculated only for the achievement-related items, as these were most relevant to the exam stressor. The CSQ achievement subscale correlated with the CSQ total score at r = 0.93 (CI [0.88 0.96], p < 0.001); alpha for achievement-related NCS was 0.89.
Finally, at this visit participants also completed the Multiple Affect Adjective Checklist–Revised (MAACL–R; Zuckerman & Lubin, 1985), a checklist comprising 132 affective words (e.g., “satisfied,” “tormented,” “inspired”). Participants are asked to check all boxes that describe their emotions “right now, today” and to “work rapidly.” Subsets of words are summed to create the subscales for depression and anxiety (other scales were not examined for this study), which had alphas = 0.71 and 0.69, respectively.
During the MRI study visit, prior to the scan, participants again completed the BDI–II (alpha = 0.90) and the MAACL (alphas = 0.65 and 0.61) to assess change in symptoms. They additionally completed the Particular Inferences Questionnaire (PIQ; Metalsky, Halberstadt, & Abramson, 1987) a four-item questionnaire assessing negative inferences about a specific event (in this case, the midterm exam). Students were asked to write down a single cause for their midterm grade and then respond to one question each about the globality of the cause, the stability of the cause, anticipated negative consequences from the exam and its impact on the student’s self-worth. All responses were on a 7-point Likert scale. Alpha = 0.57. Finally, they completed an unvalidated questionnaire with questions about grade aspiration, grade received and feelings about grades on a 1–7 scale (1 = the best I’ve ever felt, 7 = the worst I’ve ever felt).
FMRI Task
The Working Memory Task (WMT) was adapted from Joormann et al. (2011). The overall aim of this task was to have students engage in working memory manipulation of domain-general (negative) and domain-specific (exam-related) words.
Stimuli for this task came from several sources. Negative words were selected from the Affective Norms for English Words (ANEW) database (Bradley & Lang, 1999). Exam-related words were selected from course textbooks, then cross-referenced with the syllabus to ensure selected words were relevant to the most recent midterm. Neutral words were selected from the SUBTLexus database (Brysbaert & New, 2009), which was also used to provide frequency data for all words. Examples of negative, neutral and exam-related words are provided in supplemental material. 48 words were selected in each category (negative, neutral and exam-related) and normed such that average frequency and length did not significantly differ between categories. An additional 288 un-normed neutral words were selected as foils. Because the course had four sections and the study was conducted after two different midterm exams, a total of 8 versions of the task were created, with different exam-related words but identical negative and neutral words and foils.
The task comprised 144 trials divided into 48 negative, 48 neutral and 48 exam-related trials. The trial structure is outlined in Figure 1. On each trial, a participant was presented with three words for one second each, followed by a fixation cross for either 500 ms (first two words) or jittered between .25–1.25 s (following the third word). The words and inter-word fixations comprised the word epoch, which had its onset at the start of the trial and duration of 4s. Participants were then presented with a cue asking them to remember those words in either forward or backward order (.25–1.25 s jittered), followed by a 3.5-s rehearsal epoch. Finally, they were re-presented with one of those words and asked to respond (within 1.5 s) which number it was in that order. For instance, if they saw the words “psychology, tree, pencil” and the cue “backward,” then the word “psychology,” they would respond with the number “3.” On each trial, one of the three words was a critical word—either a negative, exam-related or neutral word, as described above. The remaining two words were randomly drawn without replacement from the pool of neutral foils.
Fig. 1.
Schematics of (a) the study recruitment procedure and (b) the Working Memory Task.
All statistical analyses for the behavioral data were performed using R software (https://cran.r-project.org/). Sensitivity scores (d’) were derived from the responses using a 3-alternative forced choice method (Stanislaw & Todorov, 1999) as implemented in the sensR package (https://CRAN.R-project.org/package=sensR). Trials with missing responses were dropped. Reaction times were analyzed only for correct trials. Reaction times and sensitivity were averaged across participants over a given study condition, e.g., negative trials. Contrasts (e.g., negative vs. neutral) were tested by subtracting one condition from another and testing the difference scores either as t-tests or in correlation or regression models with individual difference variables of interest, especially CSQ scores.
FMRI Data Collection and Analysis
Image acquisition
Structural and functional images were collected on a 3T MRI scanner (Discovery MR750, General Electric Medical Systems) with an 8-channel radio-frequency (RF) head coil array. T1-weighted structural images (1 mm3 voxels) were acquired in the sagittal plane with an isotropic BRAVO sequence using a parallel imaging factor of 2 (TR=6.7, TE=2.93, TI = 450, flip angle = 12°, voxel size = 1mm3, matrix size 256×256). Functional data were collected as T2*-weighted gradient-echo echo-planar images with a parallel imaging factor of 2 (TR = 2000, TE = 20, flip angle = 75°, voxel size = 3.5×1.75×1.75mm, matrix size 96×64, 40 interleaved sagittal slices). Six runs of 5 minutes 34 seconds (167 volumes) were collected. During the scan, participants wore a respiration belt, a pulse oximeter and skin-conductance electrodes on their left hands. Participants held a button-box with their right hands, which they used to respond to the working-memory task.
Preprocessing
T1-weighted images were skull-stripped using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). All other image processing was completed using FSL (www.fmrib.ox.ac.uk/fsl). Using the FEAT tool, we removed the first five volumes from each subject’s functional data, then performed motion correction using MCFLIRT and removed non-brain regions using BET. Subjects’ EPI data were aligned to their structural data using a 6 degree of freedom boundary-based registration (Greve & Fischl, 2009) and the structural MRI was registered to MNI space using a 12 degree of freedom affine transformation in FLIRT (Jenkinson & Smith, 2001) and then further refined using FNIRT nonlinear registration (Andersson, Jenkinson, & Smith, 2007). Data were smoothed with a 5-mm FWHM kernel.
FMRI data analysis
Task data were analyzed using a general linear model. Regressors were constructed at the first level by convolving boxcar functions with a double-gamma canonical HRF. The following regressors were included for each task run: word (4s), fixation (jittered .25–1.25s), cue (jittered .25–1.25s), rehearsal (3.5s), probe (1.5s), and rest (3s). In order to control for the potential confound of response time, additional regressors were constructed for the probe with trial-by-trial amplitude modulation by response time (mean-centered per condition) (Mumford & Poldrack, 2014). Regressors prior to the cue were divided into negative, neutral, exam-related, and missed trials (excluded), while regressors including and following the cue were additionally divided into forward and backward. These task-related regressors totaled 42 (see Figure S1). Additional regressors were included to model standard and extended motion parameters (24 total) and to exclude any TRs with framewise displacement > 0.9mm (0–40 regressors per run, average = 2)2. Runs were excluded if >25% of TRs were labeled as having high motion; only one run for one participant was excluded for this reason. 2 participants had 4 runs of data dropped due to falling asleep or a projection error; all other participants had all 6 runs included.
Contrasts were constructed at the first level by subtracting a regressor in one condition (e.g., neutral words) from the same regressor in another condition (e.g., negative words). Differences were not seen between forward and backward trials for any trial type, so forward and backward regressors within a given condition were combined (e.g., [forward+backward negative rehearsal] – [forward+backward neutral rehearsal]). Trial type-by-time interactions were modeled as within-trial contrasts, e.g. 2 (negative vs. neutral) × 2 (rehearsal epoch vs. word epoch). Contrast estimates were averaged across runs using a fixed effects linear model, and the subject-specific estimates for both mean estimates and correlations with behavioral measures (e.g., the CSQ) were modeled at the group level using FLAME. Multiple comparison correction was done using cluster-based random field theory with a cluster forming threshold of z = 3.1 (Eklund, Nichols, & Knutsson, 2016) and a corrected cluster p-value threshold of 0.05.
Analyses were conducted both including and excluding the four participants who received their desired exam grades; as results did not differ appreciably, these students were included for all reported analyses. Additionally, correlations with CSQ score as predictor variable were also tested as regressions with interaction between CSQ score and 1) date from exam and 2) difference between desired and actual exam grade. These interactions were all nonsignificant, and including these terms as covariates did not change reported outcomes appreciably, so these analyses are not reported and participants are treated as a single post-stress group.
Electrodermal activity
During MRI data collection, participants wore Biopac™ (www.biopac.com) MRI-compatible sensors on the middle and ring fingers of their left hands. Data were collected via a Biopac MP150 amplifier and recorded on Acqknowledge software (sampling rate = 1000 Hz). Data were downsampled to 1Hz and high-frequency noise was removed using wavelet functions in Matlab (www.mathworks.com). Areas of artifact were hand-scored and replaced using linear interpolation with in-house code. Cleaned data were analyzed using the Continuous Decomposition Analysis module in ledalab (www.ledalab.de), which produced estimates of skin conductance during the word-presentation and rehearsal epochs as defined above. These were treated as unthresholded continuous variables, i.e., averaged across all runs for a given condition (e.g., negative) and subjected to paired t-tests and correlations with individual difference variables of interest.
Results
Questionnaire data
NCS and Mood Symptomatology
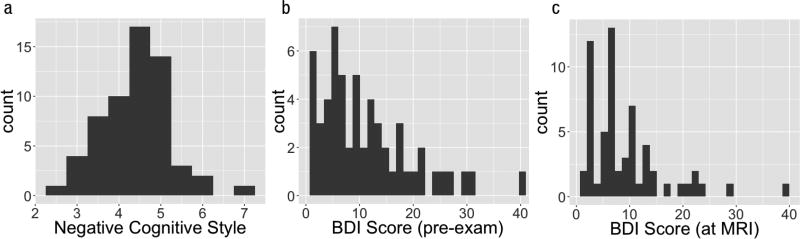
First, we examined the distribution of negative cognitive style and its relationship to depressive symptomatology. Mean score on the negative items of the CSQ (i.e., negative cognitive style) was 4.42 (SD = 0.78, range = 2.62–6.93; Figure 2). CSQ score did not differ by gender, nor was it correlated with grade on the exam, high school GPA or ACT scores. Mean BDI-II score on the online questionnaire was 11.28 (SD = 8.46, range = 1–40). Mean BDI-II score at the MRI time point was 9.08 (SD = 7.31, range = 1–39). Mean MAACL depression on the online questionnaire was 1.18 (SD = 1.64, range = 0–6) and MAACL anxiety was 1.67 (SD = 1.84, rage = 0–7). At the MRI timepoint, mean MAACL depression and anxiety scores were, respectively, 0.62 (SD = 1.15, range = 0–5) and 0.87 (SD = 1.29, range = 0–7).
Fig. 2.
Histograms of (a) CSQ score for negative scenarios, i.e., negative cognitive style; (b) depressive symptoms (BDI score) at the in-person questionnaire session, and (c) at the MRI timepoint.
As expected, BDI score was correlated with CSQ score both on the online questionnaire (r = 0.66, CI [0.48, 0.79], p < 0.01), and at the MRI time point (r = 0.48, CI [0.25, 0.65], p < 0.01). Likewise, MAACL depression score correlated with CSQ at both time points (online questionnaire: r = 0.50, CI [0.28, 0.67], p < 0.01; MRI: r = 0.35, CI [0.11, 0.56], p < 0.01). CSQ was correlated with MAACL anxiety score on the online questionnaire (r = 0.37, CI [0.12, 0.57], p < 0.01) but not at the MRI time point (r = 0.22, CI [−0.04, 0.45], p = 0.10). Surprisingly, higher CSQ score was associated with a larger decline in both BDI and MAACL depression scores pre-to-post midterm (BDI: β = −2.46, CI [−3.98, −0.94], p < 0.01; MAACL: β = −0.52, CI [−1.03, −0.01], p < 0.05).
Reactions to the Exam
Second, we assessed reactions to the exam and whether these were associated with NCS. Participants’ mean reaction to the exam on our single-item measure was 4.3 out of 7 (SD = 1.43, range = 1–7). A score of 4 corresponded with “Neutral – neither good nor bad,” indicating that on average participants felt slightly worse than neutral about their exam performance. Feelings about the exam were uncorrelated with CSQ score, indicating that participants with NCS did not feel worse about their exam performance than others. They did, however, make more negative inferences about the exam, as indicated by the correlation between PIQ scores and CSQ scores (r = 0.50, CI [1.46, 3.97], p < 0.001). Average PIQ score was 11.2, SD = 4.29, range = 3–21.
Behavioral and fMRI correlates of negative cognitive style
Correlations of CSQ total scores with behavioral data
We predicted that at-risk individuals would make more errors on the Working Memory Task. Contrary to our predictions, CSQ scores did not correlate with reaction time or sensitivity (d’) for any trial type. RT and d’ values by condition are available in Supplemental Information.
Correlations of CSQ total score with fMRI activity
Our primary question with respect to fMRI data was whether individuals with NCS had increases in fMRI activity to negative or exam-related words. In order to test this, we produced BOLD fMRI estimates for the contrasts of negative > neutral trials, exam-related > neutral trials, and exam-related > negative trials in the word presentation and rehearsal epochs. We hypothesized that the word presentation epoch, wherein participants were presented with words initially, might capture attentional or semantic processing, while the rehearsal epoch, when participants were tasked with keeping the words in working memory, might capture ruminative processing. Linear regression was then performed using each of these contrasts as the dependent variable and CSQ as the regressor of interest.
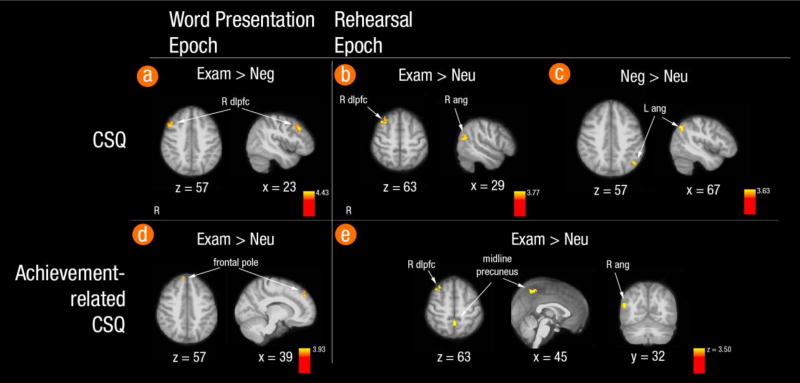
Within the word-presentation epoch, no statistically significant correlations were seen between CSQ scores and activity in exam > neutral or negative > neutral contrasts. However, there was a positive correlation between CSQ scores and the exam > negative contrast in one cluster in the right middle frontal gyrus (Figure 3 panel (a); 168 voxels, peak voxel at 50, 18, 42 mm). Thus, individuals with NCS recruited more DLPFC activity in response to exam-related than negative words during word presentation.
Fig. 3.
Whole-brain activity correlated with the CSQ during the Working Memory Task: (a) for exam-related > negative trials the word presentation epoch; (b) for exam-related > neutral trials in the rehearsal epoch; (c) for negative > neutral trials in the rehearsal epoch; and correlations between achievement-related CSQ items and brain activity for (d) exam-related > neutral trials in the word presentation epoch and (e) exam-related vs. neutral trials in the rehearsal epoch. Clusters produced using FLAME1 with z = 3.1 and a corrected cluster threshold of p < .05. Background for overlay is the average structural image warped into MNI space. Abbreviations: dlpfc = dorsolateral prefrontal cortex; ang = angular gyrus.
Within the rehearsal epoch, CSQ score positively correlated with the exam > neutral contrast in two clusters in right middle frontal gyrus and angular gyrus (Figure 3 panel (b); 180 voxels, peak voxel at 40, 24, 50 mm; 97 voxels, max at 52, −60, 22 mm). Thus, individuals with high CSQ scores had more activation for exam-related than for neutral trials in these two clusters during rehearsal. The negative > neutral contrast was positively correlated with CSQ score in one cluster in left angular gyrus (Figure 3 panel (c); 85 voxels, peak voxel at −44, −66, 46 mm), indicating that individuals with NCS recruit angular gyrus when rehearsing negative information. No statistically significant correlations were seen for the exam > negative contrast in the rehearsal epoch, indicating that individuals with NCS did not process these word types differently.
Trial type-by-epoch interactions
The activation patterns that were correlated with CSQ score differed between word presentation and rehearsal epochs, so we constructed trial type-by-epoch interactions and regressed these onto CSQ scores to test whether CSQ-related BOLD activity was greater in the word presentation or the rehearsal epoch. None of the interactions tested (word presentation vs. rehearsal for negative > neutral, exam > neutral, and exam > negative contrasts) were significant. This indicates that we were unable to detect changes in brain activity between the word-presentation and rehearsal epochs of the task.
Correlations with the CSQ achievement subscale
Whole-brain correlations with CSQ were repeated using only the achievement items on the CSQ, which are the most relevant to the stress of poor midterm performance and thus more sensitive to stress-related brain activity. During word presentation, achievement-related CSQ scores were positively correlated with the exam > neutral contrast in a cluster in frontal pole (Figure 3 panel (d); 78 voxels, peak voxel at 16, 44, 34 mm), which was not seen using the full CSQ. In the rehearsal epoch, achievement-related CSQ score was positively correlated with exam > neutral activation in right angular gyrus and middle frontal gyrus clusters as seen for the full CSQ (114 voxels, peak voxel at 38, 22, 50 mm; 66 voxels, peak voxel at 52, −62, 22 mm), as well as with a cluster in dorsomedial precuneus (67 voxels, peak voxel at 0, −54, 54 mm) (Figure 3 panel (e)). Thus, individuals with higher achievement-related NCS had more fMRI activity to exam-related words in the frontal pole during word presentation and in precuneus, R angular gyrus and middle frontal gyrus during rehearsal. No clusters survived thresholding in the negative > neutral contrast or exam > negative contrasts.
Correlations with depressive symptoms
Because CSQ scores were highly correlated with depressive symptoms, we conducted additional analyses to determine whether the correlations between CSQ and BOLD activity held when controlling for symptomatology. No clusters emerged from voxelwise correlations between BOLD activity and depressive symptoms from the BDI-II at the MRI time point. There were likewise no significant results using difference in BDI score pre-to-post midterm. Finally, we ran multiple regressions with CSQ score controlling for BDI score. In these analyses, the partial correlations between CSQ score and brain activity in the negative > neutral rehearsal period were no longer significant, but the DLPFC cluster from the exam > neutral rehearsal period remained significant.
Mean effects
In order to test whether negative and exam-related words are processed similarly regardless of individual variance in NCS, we tested mean differences between conditions. During the word-presentation epoch, one cluster in left angular gyrus was more active for negative than neutral trials (135 voxels, peak voxel MNI coordinates −60, −62, 26 mm), and one cluster in right precentral gyrus was more active for exam-related than neutral trials (168 voxels, peak voxel at 58, −2, 30 mm). No differences were seen for the exam-negative contrast. During the rehearsal epoch, no significant differences were found in any of the contrasts examined (negative-neutral, exam-neutral, exam-negative). These findings suggest that increased fMRI activity is used to process exam-related and negative words compared to neutral words normatively.
Electrodermal Activity
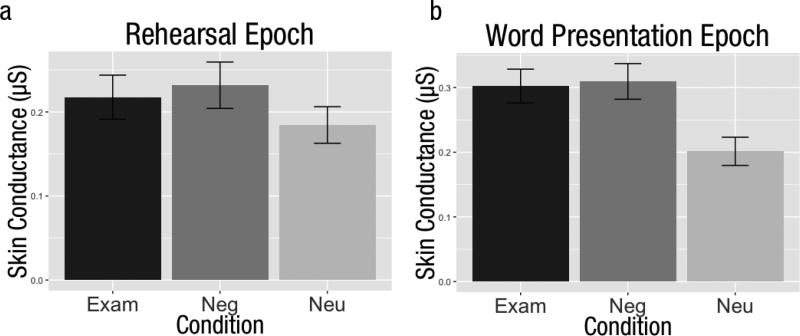
EDA scores were greater for negative than neutral trials both during the word-presentation epoch (t(59) = 5.53, CI [0.07, 0.15], p < 0.01) and the rehearsal epoch (t(59) = 3.33, CI [0.02, 0.08], p < 0.01). Likewise, EDA responses were greater for exam-related than neutral trials during the word presentation epoch (t(59) = 4.78, CI [0.06, 0.14], p < 0.01) and the rehearsal epoch (t(59) = 2.79, CI [0.01, 0.06], p < .01). However, EDA did not differ between exam-related and negative trials during either the word presentation or rehearsal epochs (both p > 0.26), and no measure of EDA correlated with CSQ score. Voxelwise brain data in the above contrasts was regressed onto EDA data and no clusters were found to be significant. In addition, when EDA scores were included as covariates in voxelwise correlation analyses with CSQ scores, the clusters reported above for negative-neutral and exam-neutral rehearsal periods were unchanged. These results indicate that differences in brain activity correlating with negative cognitive style were not attributable to arousal (Figure 4).
Fig. 4.
Differences in skin conductance response between task conditions for the (a) word presentation and (b) rehearsal epochs. Error bars represent +/− 1 standard error of the mean for that condition (e.g., negative).
Discussion
Stressful events are ubiquitous in life, and individuals who respond to them with maladaptive attributions are at increased risk for psychopathology. The central question of the present study was whether this tendency—negative cognitive style—corresponds with detectable cognitive or fMRI differences after such an event. In fact, individuals with NCS showed increased BOLD activity in response to stress-relevant information in frontal and parietal cortices. Furthermore, a strength of our study was the use of both domain-general, i.e. negative, and domain-specific, i.e. exam-related, stimuli. Mean differences in whole-brain analyses during the word presentation epoch indicate that these words are normatively processed differently from neutral words irrespective of negative cognitive style. However, during the rehearsal epoch, both stimulus types elicited increased frontoparietal activity in individuals with NCS, especially in angular gyrus. Within the word presentation epoch, individuals with NCS used more DLPFC to process exam-related than negative words. Taken together, these results elucidate a previously-undescribed frontoparietal network associated with stress-related processing in NCS, and indicates that within this network, domain-specific information elicits more activity than domain-general information. These results, thus, provide support for our hypothesis that domain-specific information would more reliably affect cognition than domain-general information, and supports the use of such stimuli in future neuroimaging studies of cognitive vulnerability.
Deeper nuances emerged when depressive symptoms were incorporated into analyses. Although there were no first-order correlations between brain activity and depressive symptoms, when depressive symptoms were controlled, the correlation between CSQ score and DLPFC activity to exam-related words during the rehearsal epoch remained significant, while the correlation between CSQ score and angular gyrus activity to negative words in the same epoch did not. This suggests that even during the same epoch, individuals with NCS process domain-general vs. domain-specific information differently in these two different brain regions. One explanation could be that they use DLPFC for domain-specific processing regardless of level of dysphoria, but they begin to use angular gyrus to process negative information as they develop symptomatology. It additionally seems to underscore the importance of DLPFC, specifically, to processing of domain-specific stimuli in individuals with NCS.
An additional point was that analyses using the achievement-related CSQ items uncovered additional NCS-related BOLD fMRI responses in exam-related trials. The CSQ achievement subscale comprised half the items of the CSQ total scale and the two were highly correlated, so these additional findings are striking. Our interpretation is that the other half of the items, i.e., the social subscale, were conceptually less relevant to the exam-related information and thus added noise. Hence, the use of carefully-matched questionnaire instruments to probe responses to domain-specific stimuli added nuance and depth to our results.
We find it interesting that our results were not in regions associated with emotional responding, such as the amygdala, but in regions associated with higher-order associative processing. The AG, in particular, is a multifaceted region whose functions include semantic and episodic memory (Seghier, 2013). The AG clusters seen here are functionally connected with both DMN and FPN (see supplemental information). Thus, these regions may act as nodes of interaction between these two networks. Prior research shows that the FPN is active with the DMN when participants engage in internally-focused, goal-directed cognition (Spreng et al., 2010), which would be consistent with cognition driven by self-relevant, negative causal attributions. These results suggest more of a top-down mechanism, rather than immediate emotional responses as would be reflected in amygdala reactivity.
Unfortunately there were no differences in task performance or reaction time, making it difficult to ascertain the functional significance of these activations. The rationale for the use of this task is that it should capture rumination, as distraction by task-unrelated thought causes errors or delayed response times. We are left with two possible conclusions: first, the task as adapted may not have been adequately sensitive. Perhaps it was too easy, or perhaps the fMRI environment and the $100 payment were sufficient incentive to enhance performance. Alternatively, differences in brain activity might have been unrelated to rumination. In support of this latter assertion, there were no relationships between brain activity and EDA response. We expected EDA to be sensitive to ruminative processing, and the lack of relationship to NCS suggests that these individuals were not ruminating more. Thus, although domain-specific and domain-general information did differentially affect brain activity, we cannot attribute these results to differences in rumination. The lack of relationship to EDA also confirms that they are not attributable to differences in sympathetic activity from increased emotional responding, cognitive effort or attention.
Although we had hoped that looking at both word presentation and rehearsal epochs would provide temporal cues about the nature of such processing, such as whether it was more likely semantic or early attentional processing (word presentation) vs. later stimulus-independent processing (rehearsal), none of the word by epoch interactions were statistically significant. Thus, we are left with differences in fMRI activity but limited insight into their provenance. Our interpretation of these results is that individuals with NCS may process stress-relevant information against a different cognitive context or “background,” perhaps in reference to maladaptive attributions, rather than due to effortful cognitive processes such as rumination. One can imagine, for instance, that individuals with NCS might situate word processing within a more abstract, self-referent conceptual space. In other words, these results may represent a difference in quality, rather than quantity, of cognitive processing.
A critical next question is whether, or how, these BOLD responses predispose individuals to psychopathology. NCS-related brain activity did not correspond to either post-exam depressive symptoms or pre-to-post change in symptoms, and students were not followed beyond this time point. Thus, there is no evidence to suggest that these differences either cause or predict depression. However, we cannot definitively rule it out. Importantly, we do not know that this midterm was an adequate stimulus to increase depressive symptoms. BDI scores and symptomatology declined on average across the midterm, and this decline was correlated with CSQ score. Thus, we suspect that individuals with NCS were more depressed at baseline and we captured a regression to the mean in dysphoria, obscuring any relationships we might have otherwise seen.
Additionally, we know little about the role of these findings in relation to other psychiatric disorders. We conceptualized this study within the framework of the Hopelessness Theory, which posits NCS as a risk factor for depression, and the midterm exam paradigm, which is designed to assess changes in depressive symptomatology. The question of whether NCS is a transdiagnostic risk factor remains incompletely answered, with a body of research indicating that NCS is associated only with anxiety disorders that are comorbid with depression (for a review, see: Haeffel et al., 2008), while more recent work indicates that NCS is part of a core transdiagnostic cognitive vulnerability (Hong & Cheung, 2015; Hong, Lee, Tsai, & Tan, 2017). As evidence accrues for core commonality among all forms of psychopathology (Caspi et al., 2014), we recognize that the CSQ likely has some transdiagnostic potential. However, as this potential remains incompletely elucidated, we focused our study on depressive, rather than anxious or other forms of psychopathology whose relationship to NCS are less well-understood. We note that NCS was inconsistently related to state anxiety symptomatology in our study, which suggests some specificity for depression. However, much more research is needed to clarify the relationships of NCS and NCS-related brain activity to other forms of psychopathology.
We must acknowledge the limitations of our study design. Most notably, this study lacked a control group, which means we cannot conclude that our results are due to poor performance on the midterm per se. Perhaps individuals with NCS who performed well on the exam would show similar processing differences. However, we argue that even if these responses are not stress-dependent, they are still interesting, as we do not know of prior work studying the neural mechanisms involved in NCS. Similarly, because there was no pre-stressor baseline scan, we cannot definitively say that our results would not have existed prior to the scan, but again they would still be interesting. Although exam-related BOLD activity could be due to pre-existing, idiosyncratic responses to these particular words, it seems unlikely given the relative lack of response to rigorously-matched neutral words. If such activity did precede the exam, we think it more likely that this would represent neural responses to personally-relevant words because of their relationship to coursework, which would likewise be informative with regards to NCS.
Finally, it should be emphasized that our population represents a non-clinical sample. Thus, although these students carry a known risk factor for depression (NCS), and underwent a stressor that we expect to interact with this risk factor, these students did not have symptomatology at the time of the study and we did not follow them to learn if they developed depression or other forms of psychopathology. Additionally, this study used a sample of undergraduates at a competitive university, and was a largely white and female sample. We cannot say whether these results would generalize to a dissimilar population. Future studies should extend this work outside of student populations and consider stressors relevant to those populations.
In spite of these limitations, this study succeeded in demonstrating differences in brain activity relating to negative cognitive style, suggestive of differential cognitive mechanisms of processing domain-specific and domain-general stress-relevant information via a frontoparietal network. These differences provide insight into the neural mechanisms involved in negative cognitive style and a starting point for studying their role in stress responding. We hope it will stimulate future neuroimaging research on cognitive vulnerability, and that it has underscored a need to conduct such research in ecologically-valid contexts and from the perspective of psychological theories of depression.
Supplementary Material
General Scientific Summary.
Negative cognitive style is a way of thinking about stressful events that increases an individual’s risk for depression after a stressful event. In this study, we scanned undergraduate students after a stressful event—a midterm exam—and found that those with a negative cognitive style processed negative and exam-related information differently in brain areas involved in abstract, self-relevant thought. Our results shed light onto brain networks that process stress-related information differently in vulnerable individuals.
Acknowledgments
The authors would like to acknowledge the contributions of Karthik Aroor for programming help, and Maria Olaru, Kanwal Ladhani, Surekha Nadendla, and Molly Parries for data collection. This study was approved by the University of Wisconsin–Madison IRB (protocol 2016-0693: Exam Stress and Risk for Depression: MRI). This work was supported by grants from the National Institutes of Mental Health (R01 MH043454-27, PI: Davidson; F30 MH106191 01A1, PI: Westbrook), a core grant to the Waisman Center from the National Institute of Child Health and Development (P30 HD03352, PI: Messing), and the University of Wisconsin–Madison Medical Sciences Training Program.
Footnotes
Subsets of these data have previously been presented at the Society for Research on Psychopathology (2016) and the Society of Biological Psychiatry (2017)
Dr. Richard J. Davidson serves on the board of directors for the following non-profit organizations: The Mind and Life Institute and the Center for Investigating Healthy Minds, Inc.
All alphas reported in this manuscript are for the final sample, N=60.
This paragraph refers to first-level regressors which are for the within-subjects GLM. Thus, although the number of regressors is larger than the number of subjects, the degrees of freedom at the first level are determined by the number of TRs rather than the number of subjects.
Author Contributions
Cecilia Westbrook developed the study concept. All authors contributed to the study design. Cecilia Westbrook collected all data with the help of research assistants, and analyzed data with support from Elena G. Patsenko and Jeanette Mumford. Lyn Y. Abramson and Richard J. Davidson were instrumental to interpretation and presentation of results. Cecilia Westbrook drafted the manuscript and all authors provided editing support and approved the final version for submission.
Contributor Information
Cecilia Westbrook, University of Wisconsin–Madison School of Medicine and Public Health
Elena G. Patsenko, Center for Healthy Minds, University of Wisconsin–Madison
Jeanette A. Mumford, Center for Healthy Minds, University of Wisconsin–Madison
Lyn Y. Abramson, Department of Psychology, University of Wisconsin–Madison
Richard J. Davidson, Center for Healthy Minds, University of Wisconsin–Madison
References
- Abramson LY, Alloy LB, Hankin BL, Haeffel GJ, MacCoon DG, Gibb BE. Cognitive vulnerability-stress models of depression in a self-regulatory and psychobiological context. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. New York, NY, US: Guilford Press; 2002. pp. 268–294. [Google Scholar]
- Abramson LY, Alloy LB, Hogan ME, Whitehouse WG, Donovan P, Rose DT, Raniere D. Cognitive vulnerability to depression: Theory and evidence. Journal of Cognitive Psychotherapy. 1999;13(1):5–20. [Google Scholar]
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96(2):358–372. doi: 10.1037/0033-295X.96.2.358. [DOI] [Google Scholar]
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, Lapkin JB. The Temple-Wisconsin Cognitive Vulnerability to Depression Project: Lifetime history of axis I psychopathology in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2000;109(3):403–418. [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Panzarella C, Rose DT. Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2006;115(1):145–156. doi: 10.1037/0021-843X.115.1.145. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. Vol. 2 FMRIB Analysis Group of the University of Oxford; 2007. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. Vol. Technical report C-1. [Google Scholar]
- Brysbaert M, New B. Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behavior Research Methods. 2009;41(4):977–990. doi: 10.3758/BRM.41.4.977. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Moffitt TE. The p Factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2008;34(4):932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009;30(8):2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffel GJ. After further deliberation: Cognitive vulnerability predicts changes in event-specific negative inferences for a poor midterm grade. Cognitive Therapy and Research. 2011;35(4):285–292. doi: 10.1007/s10608-010-9298-y. [DOI] [Google Scholar]
- Haeffel GJ, Gibb BE, Metalsky GI, Alloy LB, Abramson LY, Hankin BL, Swendsen JD. Measuring cognitive vulnerability to depression: Development and validation of the cognitive style questionnaire. Clinical Psychology Review. 2008;28(5):824–836. doi: 10.1016/j.cpr.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1(1):293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Miller N, Haeffel GJ. Cognitive vulnerability-stress theories of depression: Examining affective specificity in the prediction of depression versus anxiety in three prospective studies. Cognitive Therapy and Research. 2004;28(3):309–345. doi: 10.1023/B:COTR.0000031805.60529.0d. [DOI] [Google Scholar]
- Hankin BL, Abramson LY, Siler M. A prospective test of the hopelessness theory of depression in adolescence. Cognitive Therapy and Research. 2001;25(5):607–632. doi: 10.1023/A:1005561616506. [DOI] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong RY, Cheung MW-L. The structure of cognitive vulnerabilities to depression and anxiety: Evidence for a common core etiologic process based on a meta-analytic review. Clinical Psychological Science. 2015;3(6):892–912. doi: 10.1177/2167702614553789. [DOI] [Google Scholar]
- Hong RY, Lee SSM, Tsai F-F, Tan SH. Developmental trajectories and origins of a core cognitive vulnerability to internalizing symptoms in middle childhood. Clinical Psychological Science. 2017;5(2):299–315. doi: 10.1177/2167702616679875. [DOI] [Google Scholar]
- Jaworska N, Yang X-R, Knott V, MacQueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry. 2015;16(7):448–471. doi: 10.3109/15622975.2014.885659. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Joormann J, Levens SM, Gotlib IH. Sticky thoughts: Depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychological Science. 2011;22(8):979–983. doi: 10.1177/0956797611415539. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: A Swedish longitudinal, population-based twin study. Archives of General Psychiatry. 2006;63(10):1113. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria initiative: Background, issues, and pragmatics. Psychophysiology. 2016;53(3):286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Metalsky GI, Halberstadt LJ, Abramson LY. Vulnerability to depressive mood reactions: Toward a more powerful test of the diathesis–stress and causal mediation components of the reformulated theory of depression. Journal of Personality and Social Psychology. 1987;52(2):386–393. doi: 10.1037/0022-3514.52.2.386. [DOI] [PubMed] [Google Scholar]
- Metalsky GI, Joiner TE, Hardin TS, Abramson LY. Depressive reactions to failure in a naturalistic setting: A test of the hopelessness and self-esteem theories of depression. Journal of Abnormal Psychology. 1993;102(1):101–109. doi: 10.1037/0021-843X.102.1.101. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Poldrack RA. Adjusting mean activation for reaction time effects in BOLD fMRI; Presented at the Organization for Human Brain Mapping; Hamburg, Germany. 2014. [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. doi: 10.1037/0021-843X.109.3.504. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network. Annual Review of Neuroscience. 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1(1):25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cerebral Cortex. 2012;22(11):2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31(1):137–149. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Yao S. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biological Psychology. 2011;88(2–3):233–242. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Lubin B. Manual for the MAACL-R: The Multiple Affect Adjetive Check List Revised. Educational and Industrial Testing Service; 1985. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.