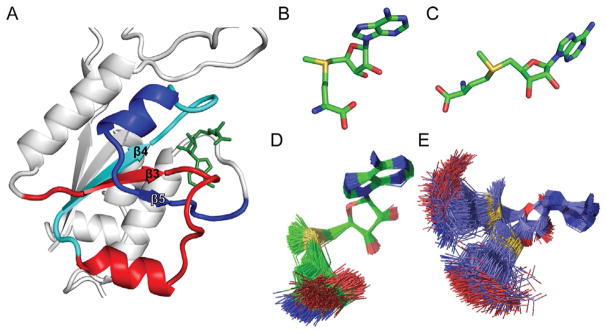
Fig. 3.
The trefoil knot structure of TrmD. (A) The trefoil knot is formed starting with the central β3 strand, which turns into β4 through a loop. The β4 makes a turn into β5, which makes a circular insertion through the loop to come out with another loop that binds the adenine base of AdoMet (PDB: 4YVI). The three β stands β3, β4, and β5, together with β1 and β2 (not labeled), form the central β sheet in the N-terminal domain of each monomeric chain in TrmD. (B) The bent conformation of AdoMet in the catalytically active monomer. (C) The open and extended conformation of AdoMet in the catalytically inactive monomer, which is similar to the structure observed in Trm5. (D) The bent conformation of AdoMet has rigid constraints and maintains the bent shape. (E) The open conformation of AdoMet has high flexibility and can extend from the bent shape to the open shape.