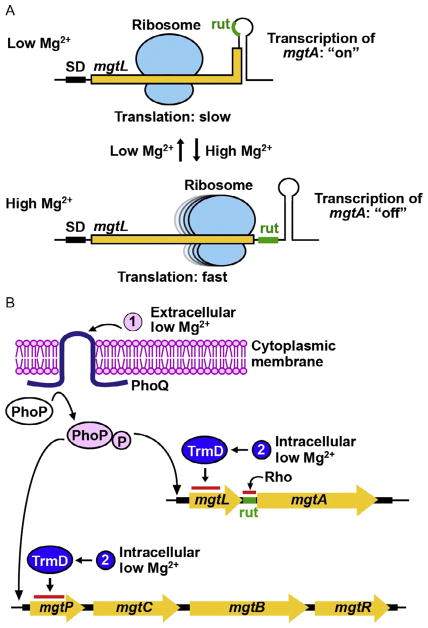
Fig. 8.
A proposed mechanism of Mg2+ sensing of Salmonella. (A) Regulation of mgtA transcription in response to Mg2+ level is mediated by the translation speed of the 5′-leader mRNA mgtL sequence, which codes for a short peptide. At low Mg2+, ribosomal translation of mgtL is slow, sequestering the rut sequence in a stem–loop structure and excluding the Rho-dependent transcription termination to permit transcription of mgtA in the “on” state. At high Mg2+, ribosomal translation of mgtL is fast, exposing the rut sequence and resulting in the Rho-dependent transcription termination of mgtA. (B) The two-component system PhoPQ provides a sensor for extracellular Mg2+. At low extracellular Mg2+, the activated PhoQ phosphorylates PhoP to turn on transcription of the Mg2+ transporter gene mgtA as well as the virulence operon mgtCBR, where mgtB is another Mg2+ transporter gene. Transcription of mgtA is then regulated by translation of the 5′-leader sequence mgtL, which encodes a small peptide. Rapid translation of mgtL exposes the Rho-utilization (rut) sequence and terminates transcription ahead of mgtA. In contrast, slow and incomplete translation of mgtL sequesters the rut sequence and allows transcription into the structural gene. The Mg2+-dependent methyl transferase activity of TrmD can act as an intracellular sensor that regulates the speed of translation of mgtL.