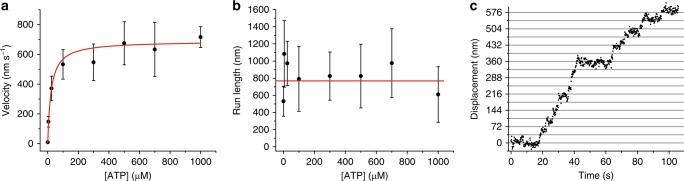
Fig. 2.
Signatures of single myosin-5B molecule motility under unloaded condition. a The velocity of myosin-5B movement (n = 127) on F-actin increases with increasing ATP following Michaelis–Menten kinetics (red curve), with vmax = 691 ± 25 nm s−1 and Kapp = 22 ± 2 µM. Error bars, s.d. b The run length (n = 127) is ATP-independent within error bars. Average run length is 780 ± 2 nm (red line). Error bars, s.d. c Representative time trace of myosin-5B stepping forward in discrete 36 nm increments on the actin filament. Average step 36 ± 4 nm (n = 42, s.e.m., [ATP] = 0.3 µM)