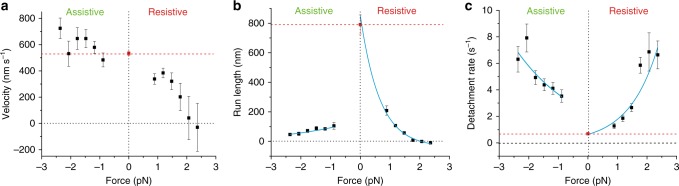
Fig. 4.
Myosin-5B velocity and run length and detachment rate from actin are force sensitive. Force dependence of myosin-5B average velocity (a), run length (b), and detachment rate (c). Detachment rate is calculated as k = 1/<τ>, where <τ> is the average run duration. In all panels, assistive forces are negative, resistive forces are positive. Red squares are the unloaded velocity (a), run length (b), and detachment rate (c) measured with the single-molecule motility assay (n = 30). Cyan curves in b and c are fits of the exponential model equations and to the data, where F is the absolute value of the force, kB the Boltzmann constant, T the temperature, and + or − superscripts indicate free parameters under positive or negative force, respectively. Fitted parameters are L0− = 148 ± 12 nm, L0+ = 890 ± 120 nm, dL− = 1.9 ± 0.3 nm, dL+ = 6.3 ± 0.6 nm and k0− = 2.3 ± 0.4 s−1, k0+ = 0.64 ± 0.09 s−1, dk− = 1.9 ± 0.5 nm, dk+ = 4.2 ± 0.4 nm. Fisher f-test applied to run length and detachment rate data under positive and negative force gave p value = 2E−5 and 6E−3, respectively, indicating significant difference of myosin-5B mechanosensitivity with force direction. [ATP] = 100 µM. n = 756. Error bars (a, b), s.e.m. (c), s.e.m. obtained from error propagation σk = στ < τ > −2. Box plots of run length and detachment rate box plot are shown as Supplementary Fig. 1a, b