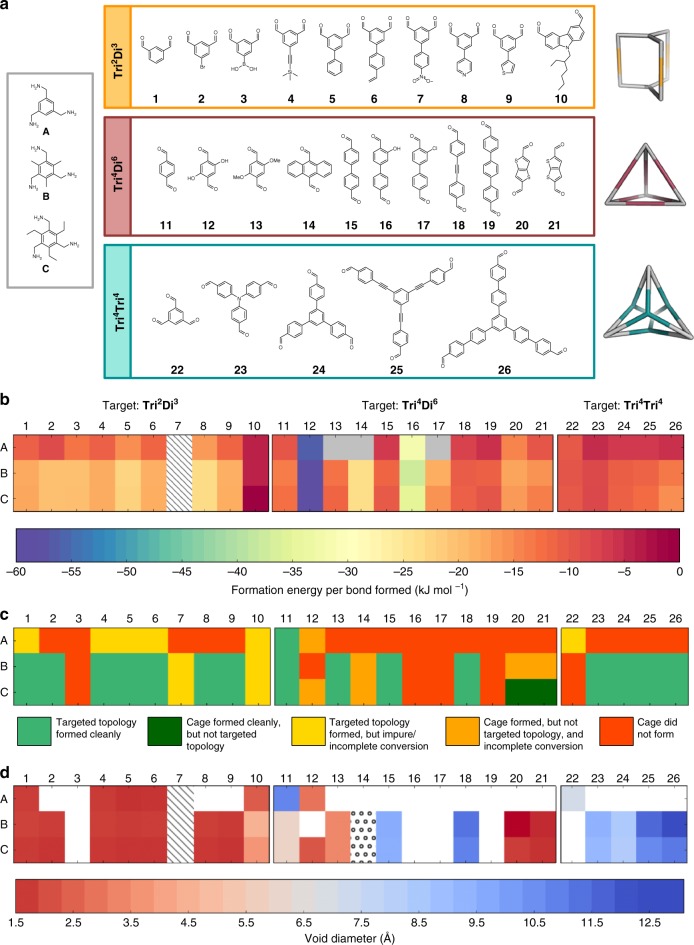
Fig. 3.
Cage precursors, target cage topologies, and results of high-throughput computation and synthesis screening. a Three triamines (A–C) were combined with three types of aldehydes to target Tri2Di3 capsules (1–10), Tri4Di6 tetrahedrons (11–21), and Tri4Tri4 tetrapods (22–26). b DFT formation energies per imine bond formed for the target cage topologies. These energies were not calculated for molecules that lacked shape persistence and collapsed (grey boxes). Cross-hatched squares denote three molecules excluded from the calculations because the force field was unreliable. c Results of the high-throughput synthesis screen. (i) light green squares—targeted cage topology was formed cleanly (31 examples); (ii) dark green squares—cage product was formed cleanly, but with topology that was not targeted (two examples); (iii) yellow squares—target topology formed but product impure or there was incomplete conversion (10 examples); (iv) orange squares—alternative topology formed and either impure or incomplete conversion (six examples); (v) red squares—no cage was formed (29 examples; 18 of these for triamine A). Cages that went against our targeted topology assumptions and formed an alternative topology are: A12, C12, C14, B20, C20, B21, and C21 (Tri2Di3 observed instead of targeted Tri4Di6) and B14 (formed a mixture of Tri2Di3 and Tri4Di6). d Void diameters as calculated from a priori computational models for cages that were formed in the subsequent reaction screen. White squares correspond to combinations where no cage was formed experimentally; squares filled with circles correspond to cages calculated to have no void