Figure 8.
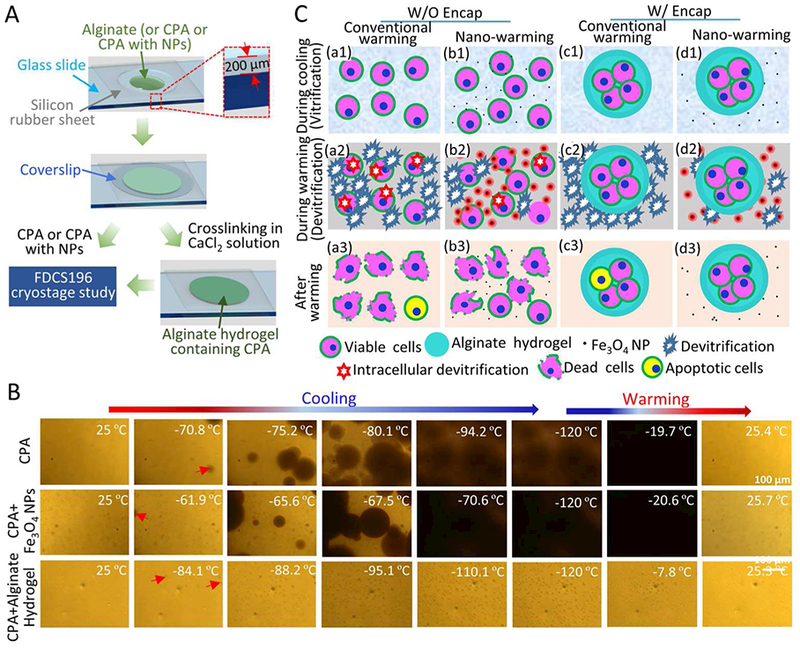
Cryomicroscopic analysis and a schematic illustration of the possible mechanisms of enhancing cell vitrification by alginate hydrogel microencapsulation and nano-warming. A) Sample preparation for cryomicroscopic studies. A silicone film and coverslip are used to keep the thickness of experimental sample, including CPA, Fe3O4 NPs with CPA, and alginate hydrogel containing CPA. B) Typical images showing the freezing and warming processes of CPA solution, CPA solution with Fe3O4 NPs, and alginate hydrogel containing CPA. The red arrows indicate initial ice crystals. For the aforementioned three samples, the ice crystals appear at −70.8 °C, −61.9 °C and −84.1 °C, and continue to grow till −94.2 °C, −70.6 °C and −95.1 °C, respectively. Recrystallization occurs at −19.7 °C and −20.6 °C during warming CPA solution and CPA solution with NPs, respectively. Recrystallization does not occur during warming of alginate hydrogel containing CPA solution. C) Cells and cell-alginate hydrogel constructs at three different stages of the vitrification cryopreservation procedure: during cooling (a1-d1), during warming (a2-d2), and after warming (a3-d3). The alginate hydrogel microencapsulation may enhance vitrification during cooling and reduce devitrification/recrystallization during warming. The latter is further minimized by nano-warming via both the selective local and global heating effect of the Fe3O4 NPs under magnetic field. The nano-warming is crucial for cryopreservation of large cell-alginate hydrogel constructs by low-CPA vitrification.
