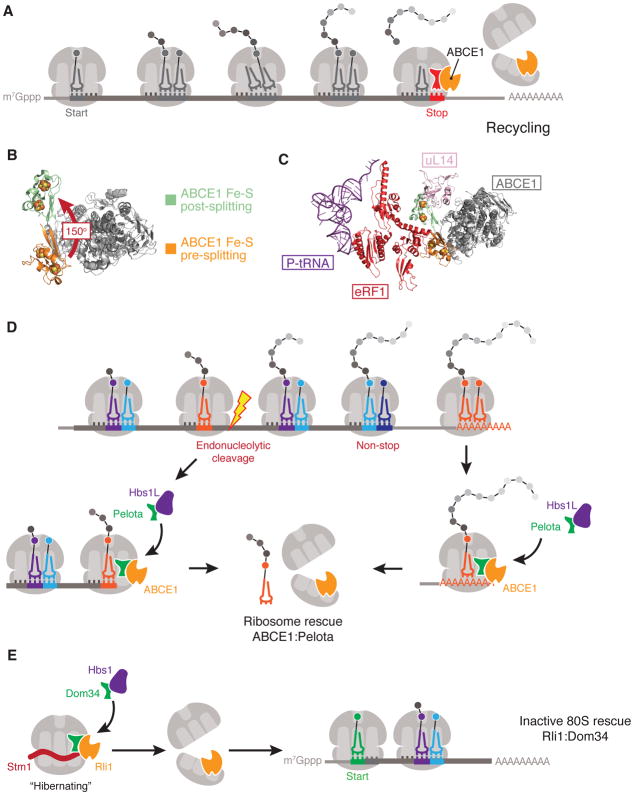
Figure 4. Ribosome recycling and rescue.
(A) Overview of ribosome recycling by ABCE1. ATP-binding cassette sub-family E member 1 (ABCE1) binds to 80S ribosomes loaded with eukaryotic peptide chain release factor subunit 1 (eRF1) and uses the power generated from ATP-binding and hydrolysis to dissociate the ribosomal subunits. ABCE1 remains bound to the 40S subunit to stimulate subsequent translation initiation steps. (B) Superposition of ABCE1 structures in the pre-splitting (PDB 3JAH) and post-splitting (PDB 5LL6) states to highlight the 150-degree rotation of the iron-sulfur (Fe–S) cluster that occurs during ribosomal subunit dissociation. (C) Superposition of ABCE1 structures pre- and post-splitting in the context of eRF1 and ribosomal protein uL14. In the pre-splitting state, the Fe–S cluster of ABCE1 interacts with eRF1. Following splitting, the Fe–S cluster undergoes a dramatic conformational change which drives eRF1 into the inter-subunit space of the ribosome to promote subunit dissociation. In the post-splitting state the Fe–S cluster would clash with 60S ribosomal protein uL14, thereby preventing 60S rejoining and effectively completing the recycling reaction. (D) Dom34–Hbs1 in coordination with ABCE1 rescues stalled ribosomes at the truncated 3′-ends of mRNAs resulting from endo- or exo-nucleolytic cleavage, or those found translating the poly(A) tail. (E) Inactive or ‘hibernating’ Stm1-bound 80S ribosomes in Saccharomyces cerevisiae can be rescued by Dom34–Hbs1 in coordination with ABCE1 to re-enter the cytoplasmic pool of translating ribosomes.