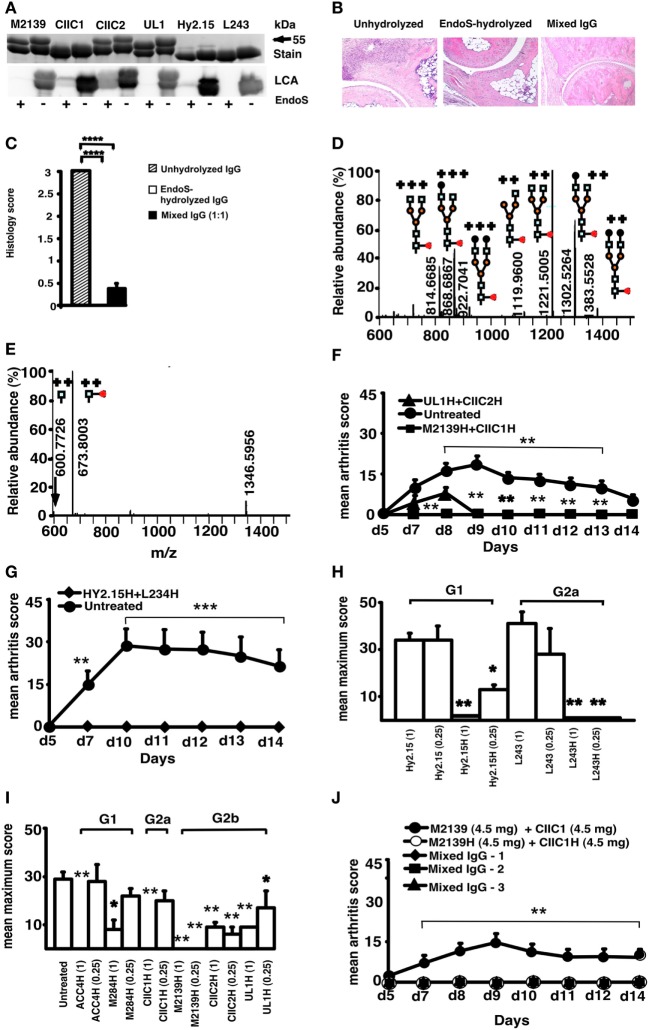
Figure 1.
Endo-β-N-acetylglucosaminidase (EndoS) and enzyme-hydrolyzed IgG inhibit inflammation. (A) SDS/PAGE and lectin blot analysis of mAbs incubated with (+) or without (−) EndoS hydrolysis and separated by 10% SDS/PAGE. The proteins were detected by PageBlue stain (Stain) or by blotting onto a PVDF membrane probed with Lens culinaris agglutinin (LCA). (B) Representative figures of H&E-stained ankle joints of mice (n = 3–4/group) injected with anti-CII mAbs; unhydrolyzed (Left), EndoS-hydrolyzed (Center), or mixed IgG (Right) on day 9. Magnification 10×. (C) Joint sections were scored under the microscope based on the scoring scale as described in Section “Materials and Methods.” In each group, at least five sections were scored for each mouse. ****p < 0.0001. Error bars indicate ± SEM. (D) Mass spectrometric analysis of Hy2.15 and (E) EndoS-treated Hy2.15. Shown spectra were acquired during the time period for which the majority of glycosylated peptides from EEQFNSTFR (21.5–23.0 min) elute. Doubly and triply charged ions as well as predicted glycan structures are shown. All numbers given are for the monoisotopic mass charge. In all of the animal experiments, male (BALB/c × B10.Q) F1 mice were used. Unless otherwise stated all of the mice received antibodies i.v. (d 0) and 25 µg of LPS i.p. (d 5). For arthritis induction in experiments shown in (F,G,J), 9 mg of two anti-CII mAb mixtures (M2139 + CIIC1) were used, whereas for experiments in (H,I) 4 mg of four anti-CII mAb mixture (M2139 + CIIC1 + CIIC2 + UL1) was used. Antigen specificity is not required for inhibition. Mice (n = 42) were injected with 4 mg of EndoS-hydrolyzed IgG (F) M2139H + CIIC1H or UL1H + CIIC2H or (G) Hy2.15H + L243H followed by anti-CII mAb. Dose and subclass dependency. (H) Mice (n = 39) were injected with EndoS-hydrolyzed or unhydrolyzed IgG1 (Hy2.15) or IgG2a (L243) mAb binding to joint unrelated antigens at two different concentrations (1 and 0.25 mg), followed by anti-CII mAb. (I) Mice (n = 65) were injected with different subclasses of EndoS-hydrolyzed anti-CII (M284H, M2139H, CIIC1H, CIIC2H, and UL1H) or anti-citrullinated CII peptide IgG (ACC4H) at two different concentrations (1 and 0.25 mg), followed by anti-CII mAb. (J) Mice (n = 25) were injected with a mixture of EndoS-hydrolyzed and/or unhydrolyzed anti-CII IgG at different combinations. In mixed IgG groups, group 1 received 4.5 mg of unhydrolyzed and 4.5 mg of EndoS-hydrolyzed IgG, group 2 had 6.8 mg of unhydrolyzed and 2.3 mg of EndoS-hydrolyzed IgG, and group 3 received 7.9 mg of unhydrolyzed and 1.1 mg of EndoS-hydrolyzed IgG. Error bars indicate ± SEM. Arthritis scoring scale of 0–60 was used (25). All the groups were compared with the untreated group for statistical analysis. *p < 0.05; **p < 0.01; ***p < 0.001.