Fig. 2.
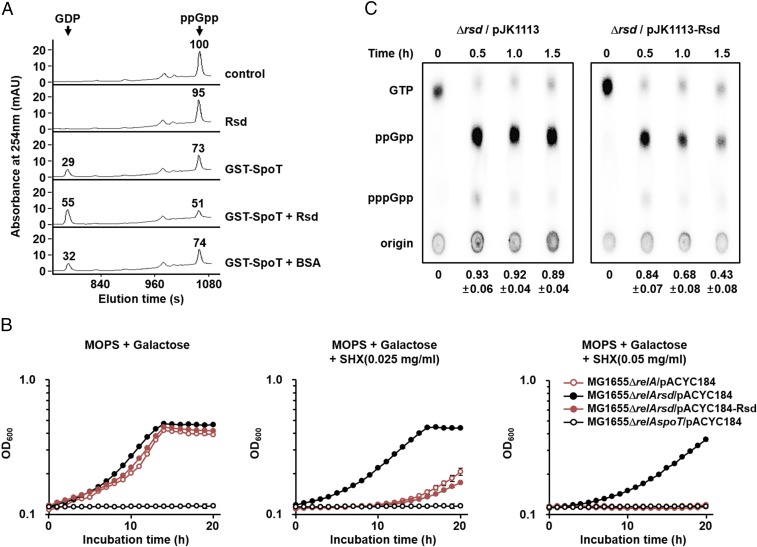
Activation of the (p)ppGpp hydrolase activity of SpoT by Rsd. (A) The stimulatory effect of Rsd on the ppGpp hydrolase activity of SpoT was measured in vitro. The ppGpp hydrolase activity of purified GST-SpoT (3 μg) was assayed in a reaction mixture containing 23.33 μM ppGpp in the presence of purified Rsd or BSA (19 μg each). After incubation at 37 °C for 5 min, the reaction mixtures were applied to a Supelcosil LC-18-T HPLC column (Sigma Aldrich), and ppGpp and GDP were monitored by measuring the absorbance at 254 nm (A254). Relative peak areas are shown above each peak, considering the peak area of ppGpp in the control sample as 100. Representative data from three independent experiments are shown. (B and C) Activation of the (p)ppGpp hydrolase activity of SpoT by Rsd in vivo. (B) Growth curves of E. coli strains in MOPS minimal medium (pH 7.2) supplemented with 0.2% galactose in the absence and presence of SHX. After inoculation, 100-μL aliquots of each strain were transferred into a 96-well plate and growth was monitored at 600 nm using a multimode microplate reader (TECAN). The mean and standard deviation of three measurements are shown. (C) The MG1655Δrsd strain carrying pJK1113 or pJK1113-Rsd was incubated in low-phosphate MOPS minimal medium containing 0.2% galactose, 0.02% arabinose, and 100 μCi/mL 32PO43−. Exponentially growing cells were treated with SHX (1 mg/mL) and intracellular (p)ppGpp concentrations were analyzed by thin-layer chromatography at the indicated times. Relative amounts of (p)ppGpp were calculated as the intensity of (p)ppGpp divided by that of (p)ppGpp plus GTP. The mean and standard deviation of three independent measurements are shown below each lane.